Uracil dermatological formulations
A uracil preparation technology, applied in the field of uracil skin drug preparations, can solve the problem of reducing curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0090] Example 1 Preparation
[0091] Prepare the four cream preparations described in Table 1 below
[0092]
[0093] Each preparation described in Table 1 above was prepared by the following procedure:
[0094] Phase A is prepared by mixing the components of phase A in an auxiliary container of appropriate size. Mix ingredients until uracil is completely dissolved. The mixture was heated slightly below 50 ° C to accelerate dissolution. The phase a auxiliary container now contains the completed phase a. In another auxiliary container of appropriate size (phase B auxiliary container), phase B is prepared by mixing the components of phase B and heating to 50-60 ℃. Mix these ingredients until most of the p-hydroxybenzoate is dissolved. Transfer phase a to phase B auxiliary container. The alkalinity of phase a contributes to the dissolution of p-hydroxybenzoate. Phase A and phase B are mixed until all components are dissolved while the temperature is maintained at 50-60 ℃. The pha...
example 2
[0096] Example 2 preliminary clinical study of uracil preparation
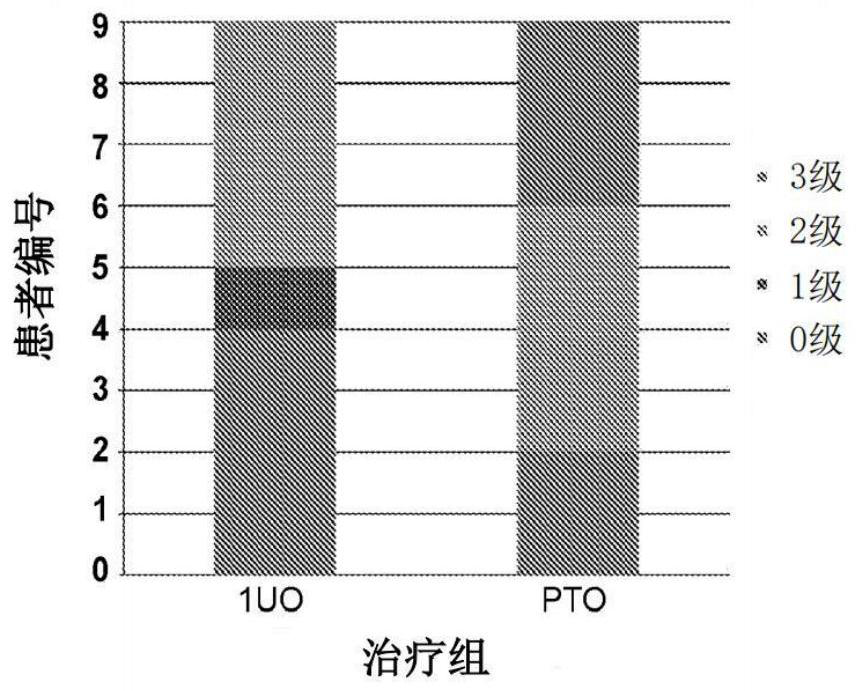
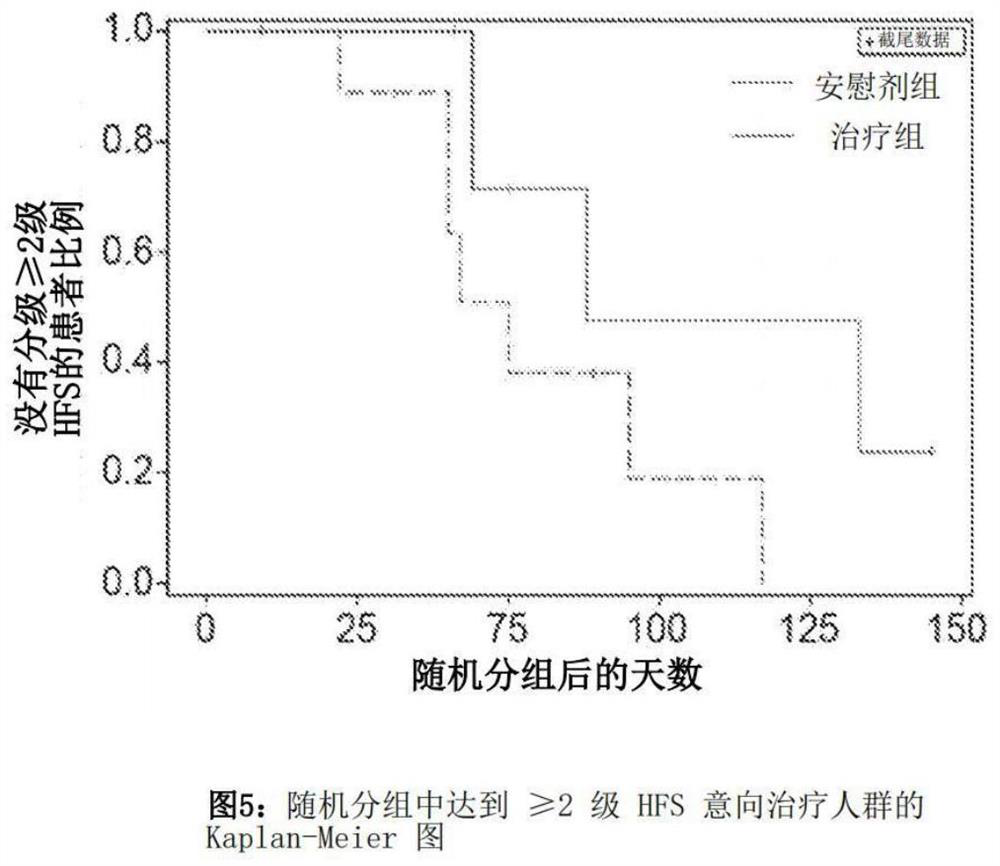
[0097] In order to evaluate the efficacy of topical administration of uracil, composition No. 2 in Table 1 above conducted a randomized, double-blind, placebo-controlled phase 1-2 clinical study in 18 patients with metastatic breast cancer undergoing capecitabine treatment. Nine patients were randomly assigned to placebo (PTO, composition 1 in Table 1). In each of the twice daily capecitabine treatments, the patient was instructed to completely rub the preparation (composition 2 (uracil) or composition 1 (placebo)) into the palms of both hands and soles of both feet twice a day. Capecitabine was administered at an approved dose of 1250mg / m on days 1-14 of each 21 day cycle 2 Oral administration twice a day. Treatment lasts up to 6 cycles unless tumor progression, unacceptable toxicity, or withdrawal of consent are recorded.
[0098] Adverse events (AES), including hand foot syndrome (HFS), were evaluated on days ...
example 3
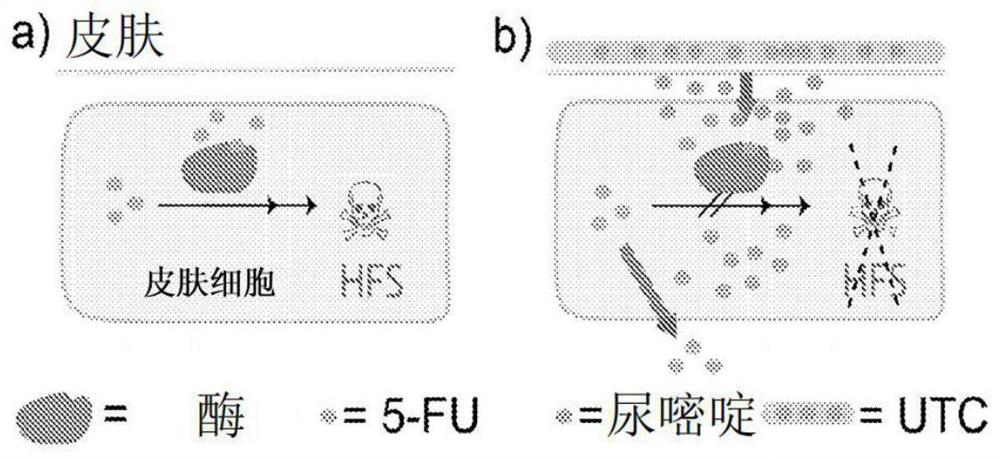
[0106] Example 3 protective effect of uracil on 5-FU in cultured human keratinocytes
[0107] Under standard culture conditions, in the presence of 10 μ M 5-FU、10 μ M 5-FU+100 μ M uracil or 10 μ M5-FU+300 μ In the case of M uracil, primary human epidermal keratinocytes (hpek cells) were cultured for 120 hours, and the cell viability was measured after 120 hours of culture. With the increase of uracil concentration, an increase in relative cell viability was observed, such as Figure 4 As shown in.
[0108] Example 4 uracil topical cream preparation and manufacturing information
[0109]Although the optimized composition in table 1o is not completely effective, it is found that the composition in table 1o is not completely effective. Therefore, several additional preparations were prepared. The preparation optimization work focuses on the development of a preparation that exhibits (1) enhanced penetration of uracil into the skin and minimal penetration into the systemic circulatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com