Novel synthesis method of capecitabine key intermediate 1,2,3-O-triacetyl-5-deoxy-D-ribose
A technology of triacetyl and a new method, which is applied in the fields of chemical industry and medicinal chemistry, can solve the problems of high acetylation temperature, difficulty in crystallization, and many by-products, and achieve the effect of high product purity, fast reaction speed, and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
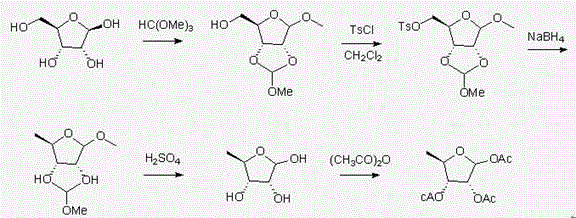
[0032]During the preparation of 1-methyl-2,3-O-methoxymethylene-D-ribofuranose, D-ribose (10.0 g, 66.7 mmol ), methyl orthoformate (2.5ml, 22.23mmol), p-toluenesulfonic acid (0.2g), stirred at 26-30°C for 4 h, tracked by TLC and liquid chromatography. After the reaction, the pH was adjusted to 8-9 with sodium carbonate, filtered, washed with dichloromethane and water, and the organic phase was rotary evaporated to obtain an oily substance, which was dried to obtain 19.7 g of white crystals with a yield of 85%. The purity by liquid chromatography test was 99.8%.
Embodiment 2
[0034] During the preparation of 1-methyl-2,3-O-methoxymethylene-D-ribofuranose, D-ribose (10.0 g, 66.7 mmoL ), methanol (50 ml), acetone (75 ml), concentrated hydrochloric acid (2.5 ml), stirred at room temperature for 19 h, followed by TLC and liquid chromatography. After the reaction, the pH was adjusted to 8-9 with sodium carbonate, filtered, washed with dichloromethane and water, and the organic phase was rotary evaporated to obtain an oily substance, which was dried to obtain 10.3 g of white crystals with a yield of 43.09%. The purity by liquid chromatography was 95%.
Embodiment 3
[0036] During the preparation of 1-methyl-2,3-O-methoxymethylene-5-O-tosyl-D-ribofuranose, add In the first step, protect the intermediate product of the reaction, then add 11.5 ml triethylamine, 0.5 g DMAP, weigh 75 ml dichloromethane, 12.5 g p-toluenesulfonyl chloride, dissolve p-toluenesulfonyl chloride with dichloromethane and drop into the reaction solution Add, ice-salt bath treatment, keep the temperature at about 5°C, and finish adding dropwise in 1 hour. An appropriate amount of triethylamine was added to keep the reaction conditions alkaline. After reacting for 12 h, wash with water and refine with isopropanol. The yield is 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com