Praseodymium-holmium-codoped lanthanum fluoride up-conversion luminescence material, and preparation method and application thereof
A luminescent material, co-doping technology, applied in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of the above-mentioned praseodymium-holmium co-doped lanthanum fluoride up-conversion luminescent material comprises the following steps;
[0029] Step S101, according to LaF 3 :xPr 3+ ,yHo 3+ The stoichiometric ratio of each element is weighed as LaF 3 , PrF 3 and HoF 3 Powder, wherein, x is 0.002~0.06, and y is 0.002~0.04.
[0030] Preferably, x is 0.03 and y is 0.01.
[0031] It can be understood that in this step, LaF can also be weighed according to the molar ratio (0.9~0.996): (0.002~0.06): (0.002~0.04) 3 , PrF 3 and HoF 3 Powder.
[0032] Step S102 , dissolving the weighed powder in hydrofluoric acid to prepare a solution with a metal cation concentration of 0.5 mol / L-3 mol / L.
[0033] The metal cation in the solution is La 3+ , Pr 3+ and Ho 3+ .
[0034] In this embodiment, the concentration of metal cations is 0.5 mol / L-3 mol / L.
[0035] Preferably, the step of dissolving the weighed powder in hydrofluoric acid to prepare a...
Embodiment 1
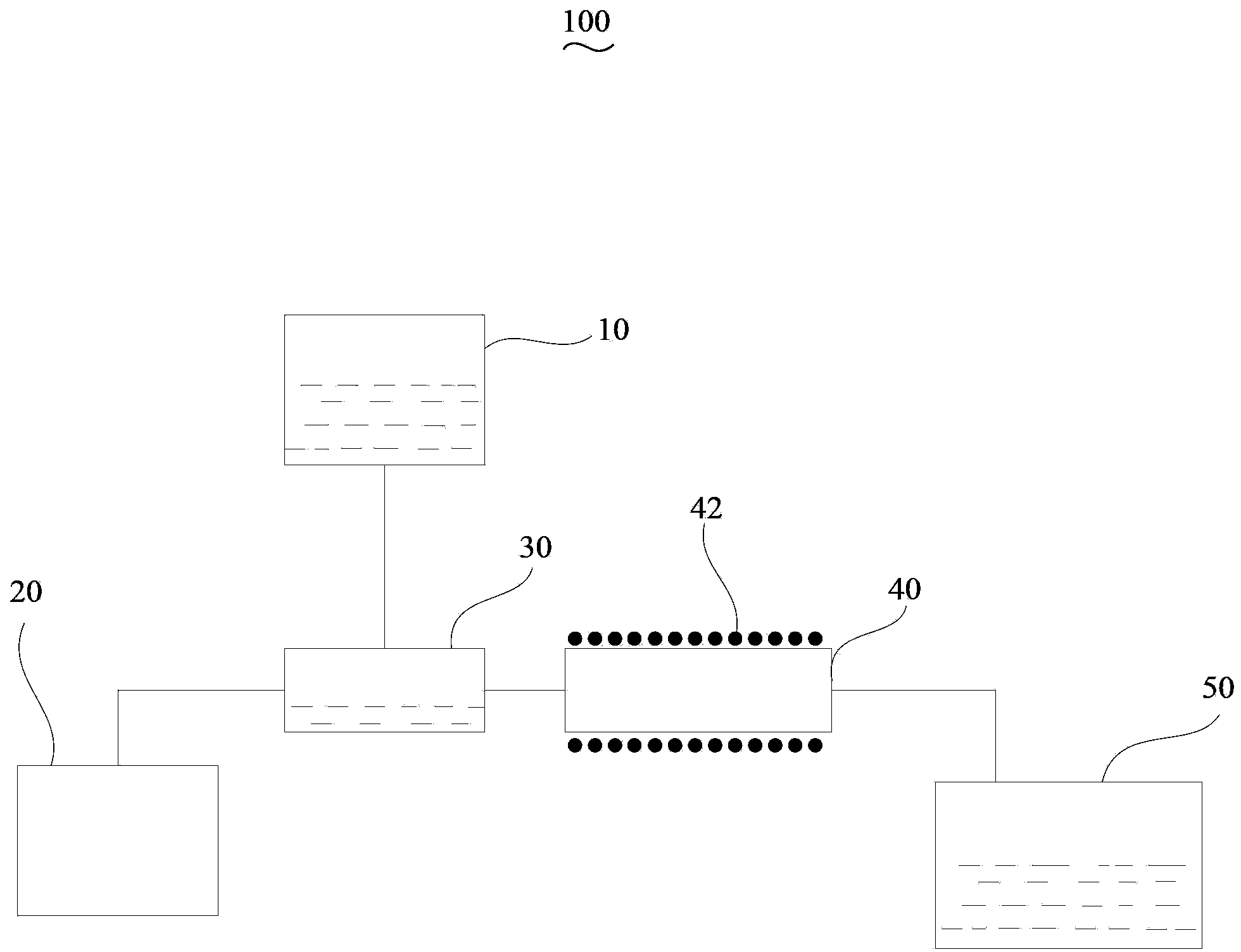
[0050] Weigh LaF 3 , PrF 3 and HoF 3 Powder, LaF 3 , PrF 3 and HoF 3 The molar ratio of each component of the powder is 0.96:0.03:0.01, dissolved in hydrofluoric acid to prepare a 1.5mol / L solution, and 0.01mol / L polyethylene glycol additive is added. Then put the solution into the atomization device, and then feed 5 L / min of argon gas into the atomization device. The solution precursor enters a quartz tube with a temperature of 180°C along with the argon carrier gas to generate the precursor, wherein the diameter of the quartz tube is 95mm and the length is 1.4m. Then the phosphor enters the condenser along with the airflow, and is finally collected by the microporous acid-resistant filter funnel. Collect the precursor of the phosphor powder, place it in a temperature-programmed furnace and calcinate for 3 hours at a calcining temperature of 1100°C to obtain LaF 3 : 0.03Pr 3+ , 0.01Ho 3+ Up-converting phosphors.
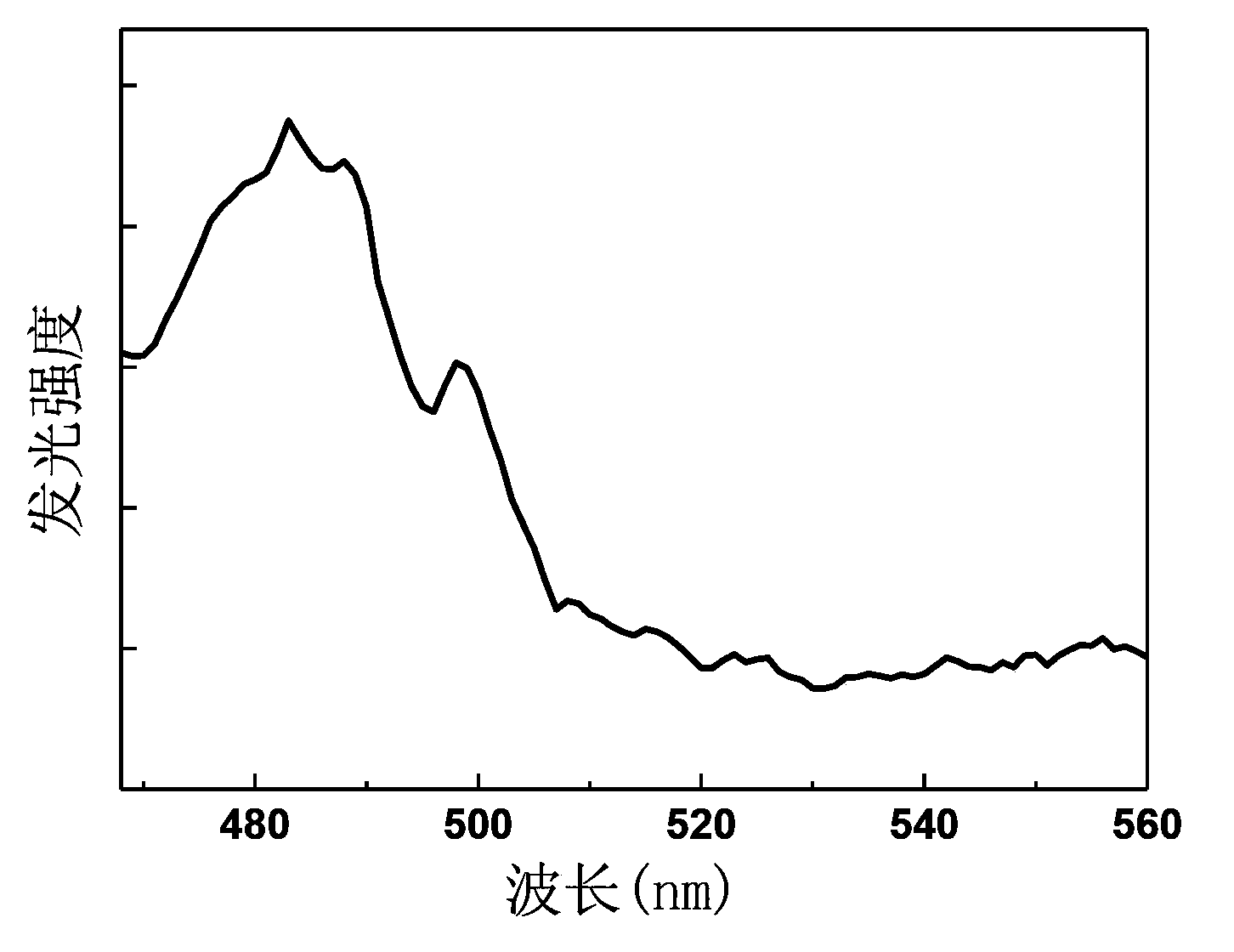
[0051] see image 3 , image 3 The middle curve 1 ...
Embodiment 2
[0054] Weigh LaF 3 , PrF 3 and HoF 3 Powder, LaF 3 , PrF 3 and HoF 3 The molar ratio of each component of the powder is 0.9:0.06:0.04 respectively, dissolved in hydrofluoric acid to prepare a 3mol / L solution, and 0.05mol / L polyethylene glycol additive is added. Then put the solution into the atomization device, and then feed 15 L / min argon gas into the atomization device. The solution precursor enters a quartz tube with a temperature of 220°C along with the argon carrier gas to generate the precursor, wherein the diameter of the quartz tube is 150mm and the length is 3m. Then the phosphor enters the condenser along with the airflow, and is finally collected by the microporous acid-resistant filter funnel. Collect the precursor of the phosphor powder, place it in a temperature-programmed furnace and calcinate it for 5 hours, and the calcining temperature is 1300°C to obtain LaF 3 :0.06Pr 3+ , 0.04Ho 3+ Up-converting phosphors.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com