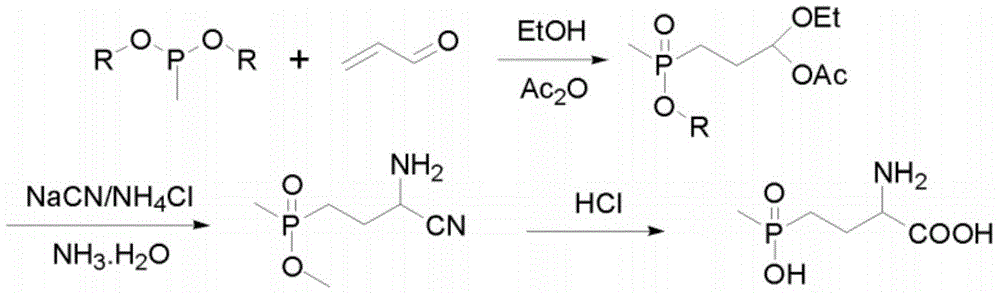
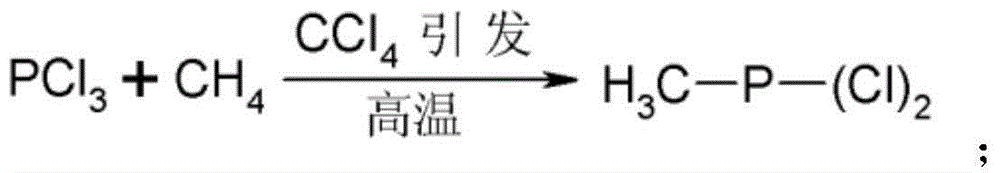
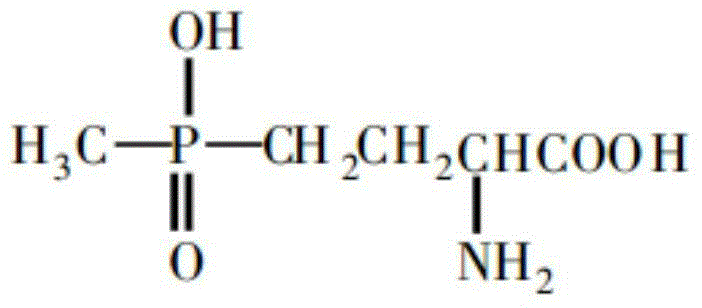A kind of method for preparing glufosinate-ammonium
A technology of glufosinate-ammonium and methyl phosphinate, which is applied in the fields of chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc. Industrial production is difficult and the reaction route is long, etc., to achieve the effects of reduced production costs, stable properties, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0037] Example 2 Preparation of isobutyl methylphosphinate
[0038] Add 300.0 mL of cyclohexane and 148.0 g (2.0 mol) of isobutanol to a 1000 mL four-necked flask, pass ammonia gas at a flow rate of 50 mL / min, stir and cool, and add 117.0 g (1.0 mol) dropwise at -10°C. A solution of methyl phosphorous dichloride and 250.0 mL cyclohexane. After dripping, react for 2 hours, filter, and remove the solvent under reduced pressure to obtain 134.2 g of a colorless liquid with a content of 96.3% of isobutyl methylphosphinate and a yield of 95.0%.
Embodiment 3
[0039] Example 3 Preparation of n-hexyl methylphosphinate
[0040] Add 400.0 mL of cyclohexane and 204.3 g (2.0 mol) of n-hexanol to a 1000 mL four-necked flask, pass ammonia gas at a flow rate of 60 mL / min, stir and cool, and add 117.0 g (1.0 mol) methyl at 0°C. A solution of phosphorus dichloride and 200.0 mL cyclohexane. After dripping, react for 3 hours, filter, and remove the solvent under reduced pressure to obtain 157.9 g of a colorless liquid, the content of n-hexyl methylphosphinate is 96.4%, and the yield is 92.8%.
[0041] The final product prepared in the following examples of the present invention is in the form of glufosinate ammonium salt, molecular formula C 5 H 18 N 3 O 4 P, the molecular weight is 215.19, the structural formula is as follows:
[0042]
Embodiment 4
[0043] Example 4 Preparation of glufosinate-ammonium
[0044] Add 55.0g (0.5mol) of ethyl methylphosphinate prepared in Example 1 to a 250ml four-neck flask under the protection of nitrogen, heat to 115°C, and add DL-2-hydroxy-3-butene dropwise A mixture of 66.3 g (0.5 mol) ethyl ester and 3.0 g n-butyric acid. The dropping time is controlled for about 2hr, and the dropping temperature is controlled at 115-120°C. After the addition, the reaction was kept for 30 minutes.
[0045] The reaction solution was cooled to about 80°C, and 37% hydrochloric acid was slowly added dropwise until the pH value reached 4, and then heated to reflux for 2hr. The acidified liquid was cooled to 40°C, the pH was adjusted to 12 with 25% ammonia water, and the reaction was kept warm for 2 hours.
[0046] The above materials are dehydrated under reduced pressure until the water content of the solution drops to 10%. Then dissolve with 300g of methanol, filter to remove impurities such as ammonium chlorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com