Method for recovering valuable metals from silver-zinc slag
A valuable metal, silver-zinc slag technology, applied in the direction of improving process efficiency, can solve problems such as unfavorable purification of bismuth chemical products, affecting the quality of electrolytic silver, harsh operating environment, etc., achieving a short and feasible process, reducing the consumption of raw materials, Work environment friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
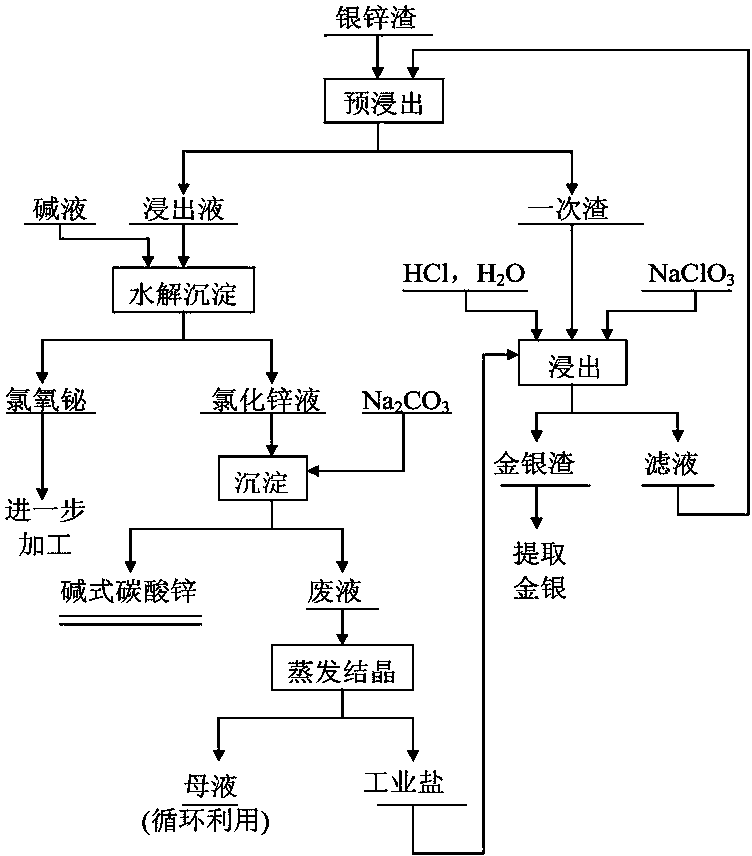
[0019] Reclaim bismuth and silver in the silver-zinc slag according to the following steps:
[0020] (1) 1000g of silver-zinc slag after ball milling, the main components of the analysis slag are: bismuth 65.21%, silver 4.18%, zinc 5.82%, lead 1.07%. Using NaClO 3 -NaCl-HCl system for leaching, the reaction temperature is controlled at 70°C, the liquid-solid ratio is 4:1, NaClO 3 The consumption is 10g / L, the consumption of NaCl is 20g / L, and the consumption of HCl is 80mL / L. After the silver-zinc slag was reacted for 4 hours, it was settled and filtered, and the leaching solution and filter residue were collected respectively. Obtain filter residue 74.65g, silver content is 60.08%;
[0021] (2) While stirring the leaching solution, adjust its pH value to 2.5 with 20% sodium hydroxide solution, stir at room temperature for 1 hour, place it in the sediment and filter, and obtain 1102 g of bismuth oxychloride filter residue containing 54.37% bismuth and zinc chloride solution...
Embodiment 2
[0025] (1) 1000g of silver-zinc slag after ball milling, the main components of the analysis slag are: bismuth 65.21%, silver 4.18%, zinc 5.82%, lead 1.07%. Using NaClO 3 -NaCl-HCl system for leaching, the reaction temperature is controlled at 75°C, the liquid-solid ratio is 6:1, NaClO 3 The consumption is 10g / L, the consumption of NaCl is 40g / L, and the consumption of HCl is 100mL / L. After the silver-zinc slag was reacted for 4 hours, it was settled and filtered, and the leaching solution and filter residue were collected respectively. Obtain filter residue 73.05g, silver content is 63.17%;
[0026] (2) While stirring the leaching solution, adjust its pH value to 3 with a 20% sodium hydroxide solution. After stirring at room temperature for 2 hours, set it aside and filter it to obtain 1089.6 g of bismuth oxychloride filter residue containing 59.64% bismuth and zinc chloride. solution. Adjust the pH value of the zinc chloride solution to 6.5 with soda ash, stir and react ...
Embodiment 3
[0030] (1) 1000g of silver-zinc slag after ball milling, the main components of the analysis slag are: bismuth 65.21%, silver 4.18%, zinc 5.82%, lead 1.07%. Using NaClO 3 -NaCl-HCl system for leaching, the reaction temperature is controlled at 80°C, the liquid-solid ratio is 8:1, NaClO 3 The consumption is 15g / L, the consumption of NaCl is 60g / L, and the consumption of HCl is 120mL / L. After the silver-zinc slag was reacted for 4 hours, it was settled and filtered, and the leaching solution and filter residue were collected respectively. Obtain filter residue 70.07g, silver content is 68.82%;
[0031] (2) While stirring the leaching solution, adjust its pH value to 2.5 with 30% sodium hydroxide solution, stir at room temperature for 3 hours, place it in the sink and filter, and obtain 1009.2 g of bismuth oxychloride filter residue containing 68.2% bismuth and zinc chloride solution. Adjust the pH value of the zinc chloride solution to 7 with soda ash, stir and react at 60°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com