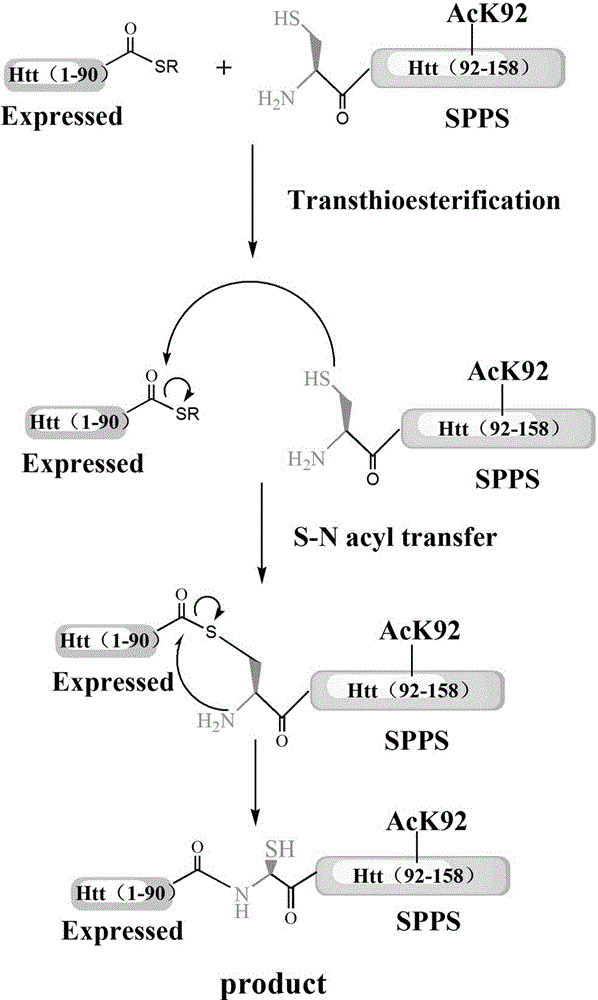
Acylation modification method of hungtintin
A technology of huntingtin protein and modification method, applied in the field of chemical modification of proteins, can solve the problems of easy aggregation and low degree of proteolysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
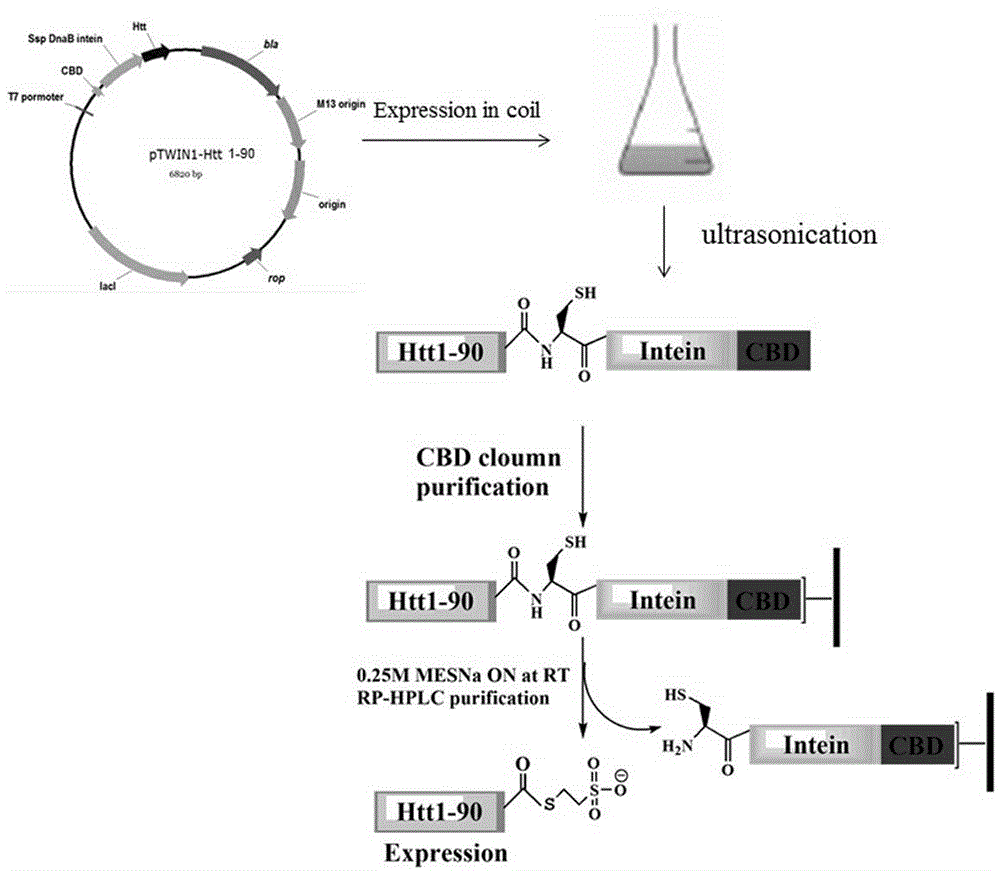
[0026] Example 1 : obtain recombinant protein Htt1-90
[0027] (1) Test materials
[0028] (1) Vectors and strains
[0029] The plasmid pcDNA3.1 / mys-His was donated by CHDI; the expression vector pTWIN1 was purchased from NEB Company; the plasmid pMD18-T and Escherichia coli JM109 and DH5α were purchased from Takara Biotechnology Co., Ltd.
[0030] (2) Primers
[0031] P1: 5-CCG GAATTC CTGCCGTGCC-3 ( Eco R?)
[0032] P2: 5-AAAA CTGCAG ACAGCCGGGC-3 ( Pst ?)
[0033] Synthesized by Shanghai Sangon Bioengineering Co., Ltd.
[0034] (2) Implementation plan
[0035] 1. Obtaining the Huntingtin Gene
[0036] Using the plasmid pcDNA3.1 / mys-His as a template, design and synthesize Eco R? and Pst ?Upstream and downstream primers P1 and P2 of restriction enzyme sites, PCR amplification htt Gene fragments, PCR products were detected by agarose gel electrophoresis and recovered, purified and ligated with the vector pMD18-T, transformed into Escherichia coli JM109, an...
Embodiment 2
[0042] Example 2 : Purification of recombinant protein
[0043] The CBD component of the Htt1-90-Intein-CBD fusion protein can be specifically combined with chitin resin to facilitate the removal of foreign proteins, and then the intein component catalyzes the fusion protein to break between Htt1-90 and Intein under certain conditions. The specific operation is as follows: the supernatant is loaded on a 2ml chitin gravity column. The column was first equilibrated with solution A (Na-HEPES (pH8.0) 20mmol / L, NaCl 500mmol / L, EDTA 1mmol / L), and the supernatant was loaded and then eluted with solution A. Then the column was soaked in solution B (Na-HEPES (pH8.0) 20mmol / L, NaCl 500mmol / L, EDTA 1mmol / L, DTT 50mmol / L) at 40C for 16h. Solution C (Na-HEPES (pH8.0) 20mmol / L, NaCl 500mmol / L) eluted protein and collected every 1ml. The samples collected at each step were analyzed by SDS-PAGE, and the purity of the identified protein was 90%.
[0044] A solution containing 0.25mmol / L p...
Embodiment 3
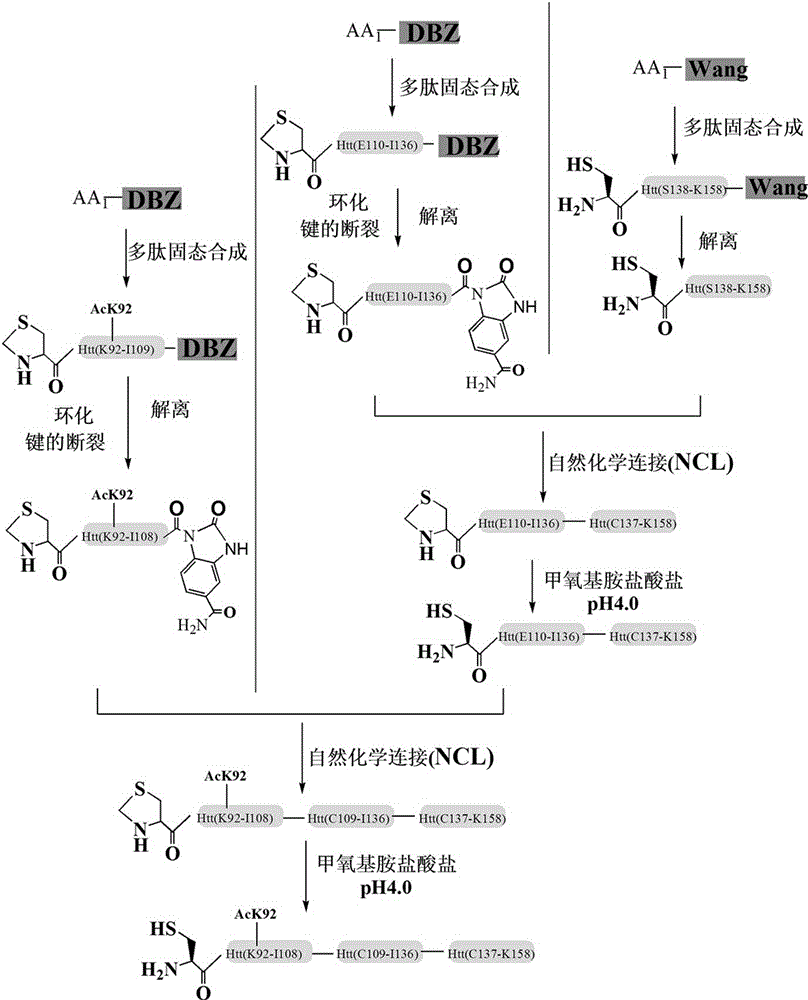
[0045] Example 3 : SPPS method to obtain Htt (Cys-K92-K158) polypeptide modified by acylation at the K92 site
[0046] The synthesis process is divided into three fragments, first synthesizing Cys-S138-K158, then synthesizing Cys-E110-L136, and finally synthesizing Cys-K92-I108. Each fragment is ligated by natural chemical ligation NCL method to obtain Htt (Cys-K92-K158) polypeptide
[0047] The synthesis of solid-phase peptides was performed on a CS336X peptide synthesizer, and the synthesis of Cys-S138-K158 peptides was based on Wang resin. The deprotection and depolymerization of the resin can proceed spontaneously in the solution of (TFA / DCM / H2O / TIPS 90 / 5 / 2.5 / 2.5). Then it was precipitated by 10 times equivalent of cold ether, and these original polypeptides were purified by reverse phase HPLC, using waters 600 pump and waters 2489 UV / visible light detector, and the column used GRACE-Vydac 218TP54 C18 column. Mobile phase A is 0.1% trifluoroacetic acid aqueous solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com