Synthetic method of alpha-hydroxyl carbonyl compound
A technology of carbonyl compounds and hydroxycarbonyl compounds, which is applied in the field of synthesizing α-hydroxycarbonyl compounds, can solve the problems such as the narrow scope of application of substrates, and achieve the effects of easy industrial production, high yield and simple conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
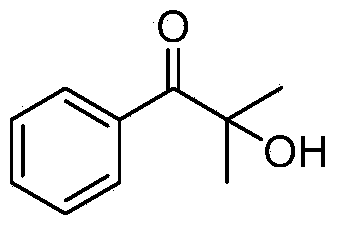
[0014] The synthesis of embodiment 12-hydroxyl-2-methyl-1-phenylacetone
[0015]
[0016] a): Take a 25mL Schlenk reaction tube, add 33mg of cesium carbonate, 74mg of 2-methyl-1-phenylacetone, 167mg of triethyl phosphite, 2mL of dimethyl sulfoxide, connect a 200mL oxygen balloon, and put it at room temperature Stir at 25°C for 24 hours. After the reaction was completed, 15 mL of ethyl acetate was added to quench the reaction, washed with 5 mL of brine, the organic phase was separated, the aqueous phase was extracted 3 times with ethyl acetate, the organic phases were combined, and separated by column chromatography to obtain 2-hydroxy-2-methyl- Pure 1-phenylacetone 71mg, yield 87%.
[0017] b): Take a 25mL Schlenk reaction tube, add 33mg of cesium carbonate, 74mg of 2-methyl-1-phenylacetone, 167mg of triethyl phosphite, 2mL of dimethyl sulfoxide, and stir at room temperature at 25°C under normal air pressure 48 hours. After the reaction was completed, 15 mL of ethyl acet...
Embodiment 2
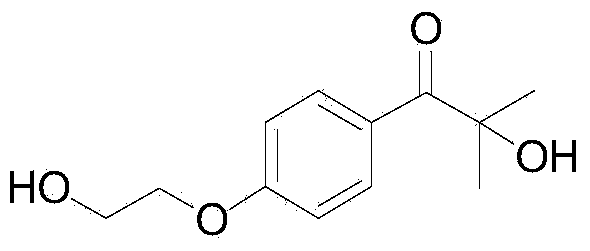
[0033] The synthesis of embodiment 22-hydroxyl-4'-(2-hydroxyethoxy)-2-methylpropiophenone
[0034]
[0035] Take a 25mL Schlenk reaction tube, add 33mg of cesium carbonate, 105mg of 4'-(2-hydroxyethoxy)-2-methylpropiophenone, 167mg of triethyl phosphite, 2mL of dimethyl sulfoxide, and connect a 200mL Oxygen balloon, stirred at room temperature 25°C for 24 hours. Add 15 mL of ethyl acetate to quench the reaction after the reaction is finished, add 5 mL of brine to wash, separate the organic phase, extract the aqueous phase with ethyl acetate for 3 times, combine the organic phases, and separate by column chromatography to obtain 2-hydroxy-4'-(2 -Hydroxyethoxy)-2-methylpropiophenone pure product 84mg, productive rate 75%.
[0036] 1 H NMR (400MHz, CDCl 3 ):δ7.97(d,J=8.8Hz,2H),6.84(d,J=8.0Hz,2H),4.04(t,J=4.4Hz,2H),3.88(t,J=4.0Hz,2H ),3.57(s,2H),1.52(s,6H); 13 CNMR (100MHz, CDCl 3 ): δ202.5, 162.4, 132.3, 126.1, 114.0, 75.9, 69.3, 60.8, 28.4 ppm; IR (neat): ν=3404.8, 2932...
Embodiment 3
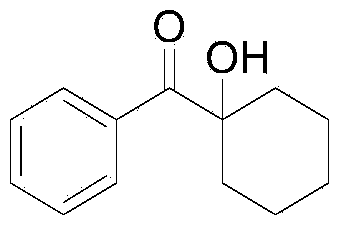
[0037] The synthesis of embodiment 3α-hydroxycyclohexyl benzophenone
[0038]
[0039] Take a 25mL Schlenk reaction tube, add 163mg cesium carbonate, 95mg cyclohexyl benzophenone, 167mg triethyl phosphite, 2mL dimethyl sulfoxide, connect a 200mL oxygen balloon, and stir at room temperature 25°C for 72 hours. Add 15 mL of ethyl acetate to quench the reaction after the reaction, add 5 mL of brine to wash, separate the organic phase, extract the aqueous phase with ethyl acetate for 3 times, combine the organic phases, and separate by column chromatography to obtain pure α-hydroxycyclohexylbenzophenone. Product 98mg, yield 96%.
[0040] 1 H NMR (400MHz, CDCl 3 ):δ8.00(d,J=7.6Hz,2H),7.53(t,J=7.2Hz,1H),7.43(d,J=8.0Hz,2H),3.40(s,1H),2.08-2.00 (m,2H),1.82-1.65(m,7H),1.39-1.29(m,1H); 13 C NMR (100MHz, CDCl 3 ): δ205.5, 135.1, 132.3, 129.4, 128.2, 78.6, 35.3, 25.3, 21.4ppm; IR (neat): ν=3460.2, 2930.8, 1675.4, 1261.9cm -1 ;MS(70ev):m / z(%):99.0(100),105(30),176.0(M + ,5).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com