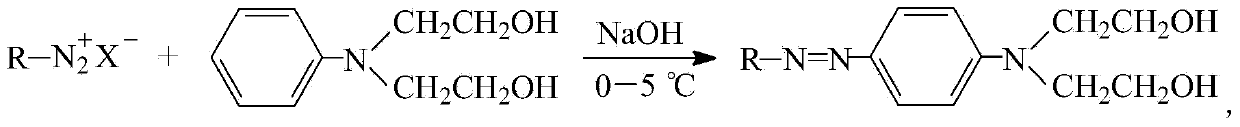Azo dibasic alcohol in symmetrical structure and preparation method thereof
A symmetrical structure and azobis technology, which is applied in the field of azodiols with symmetrical structures and its preparation, can solve the problems of few types of azodiols, low reactivity, asymmetric hydroxyl structure, etc., and achieve raw material Easy to obtain, simple process, and long-lasting photoelectric properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
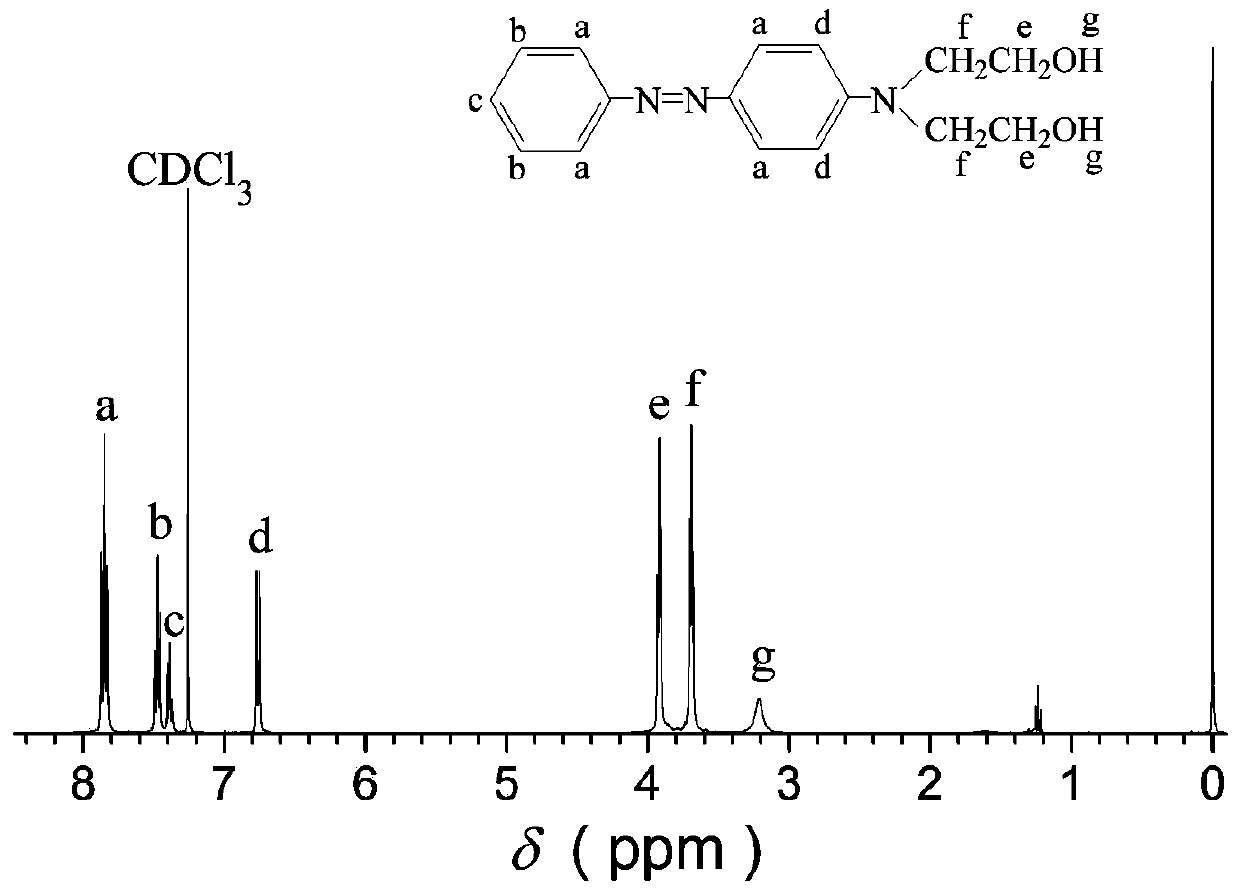
[0031] Add 3 grams (32 mmol) of aniline to 20 mL of water, then slowly add 9.8 grams of concentrated hydrochloric acid with a concentration of 37% by mass, keep the reaction system at 0-5°C, and add 2.22 grams (32 mmol) dropwise within 1 minute while stirring A solution prepared by sodium nitrite and 3.5mL water was kept at 0-5°C for 1 hour to obtain the diazonium salt solution of aniline;
[0032] Add 5 mL of water, 10 mL of methanol, and 6 mL of acetic acid into 5.8 g (32 mmol) of N,N-dihydroxyethylaniline, and stir until the N,N-dihydroxyethylaniline is completely dissolved. Keep the reaction system at 0-5°C, add the diazonium salt solution of aniline prepared above dropwise, and adjust the pH value of the system to 6 through the sodium hydroxide solution with a substance concentration of 1M, filter after 2 hours of reaction, and wash the precipitate with water Once, and then recrystallized with a mixture of ethanol and water at a volume ratio of 2.5:1 to obtain azodiol N,N...
Embodiment 2
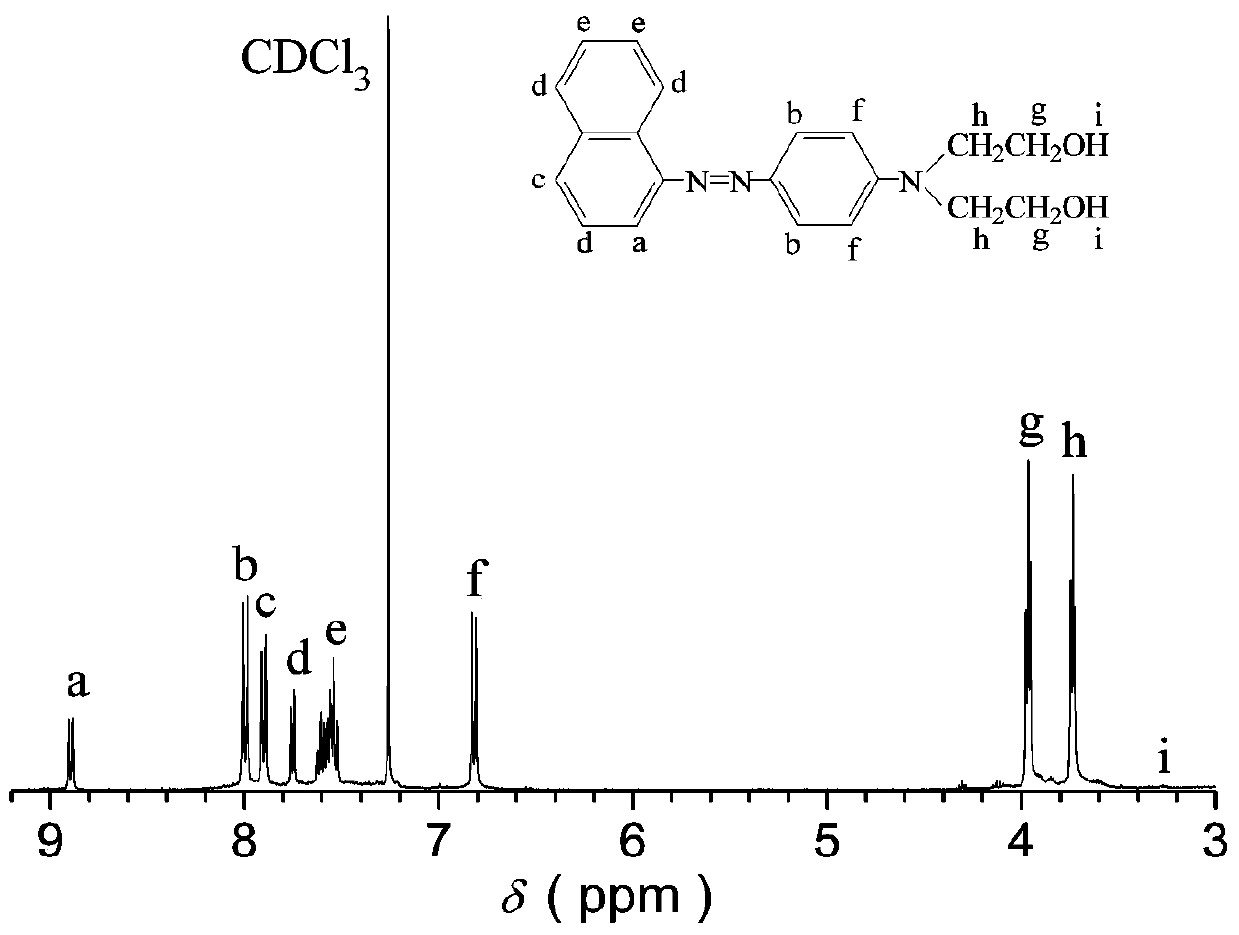
[0037] Add 3mL of acetic acid to 7.5mL of water, then add 1.5g (10mmol) of 1-naphthylamine, and then slowly add 3g of concentrated hydrochloric acid with a concentration of 37% by mass. Change to an ice bath at 0-5°C, add a solution prepared by 0.72 g (10 mmol) sodium nitrite and 1.5 mL water dropwise under stirring, and keep the reaction for 2 hours to form a diazonium salt solution of 1-naphthylamine;
[0038] Add 1mL of acetic acid and 5.7mL of methanol into 1.9g (10mmol) of N,N-dihydroxyethylaniline, keep the reaction system at 0-5°C, add the diazonium salt of 1-naphthylamine prepared above dropwise, and pass Add the amount of substance concentration and adjust the pH value of 1M aqueous sodium hydroxide solution to be 6, adjust the pH value of the system after 2 hours of reaction under magnetic stirring to be 7, filter the product and wash it with water, then use ethanol and water in a volume ratio of 5: 1 After recrystallization of the prepared mixture, azodiol N,N-dihyd...
Embodiment 3
[0044] Add 15.35 grams (127 mmol) of 4-ethylaniline to 100 mL of water, then slowly add 45 grams of concentrated sulfuric acid with a concentration of 98% by mass, stir for 5 minutes, cool down to 0-5°C, and drop the solution within 1 minute. 8.75 g (127 mmol) of sodium nitrite and 15 mL of water were prepared, and the system was kept at 0-5 ° C for 1 hour to obtain the diazonium salt solution of 4-ethylaniline;
[0045] Add 25mL of water, 24mL of methanol and 5mL of acetic acid into 23.45g (129mmol) of N,N-dihydroxyethylaniline. After forming a solution, keep the reaction system at 0-5°C, and add the above-prepared 4-ethanediline dropwise under magnetic stirring. The diazonium salt solution of base aniline, and the pH value of the system is adjusted to 6 by adding a sodium hydroxide solution with a concentration of 1M, keeping the reaction at 0-5°C for 2 hours, and then filtering, and the obtained precipitate is mixed with ethanol and water according to The mixture prepared a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com