Bifunctional organic thiosulfate and preparation method thereof
An organic thiosulfate and thiosulfate technology, applied in organic chemistry and other directions, can solve problems such as slow reaction, and achieve the effects of improving vulcanization reversion, maintaining cross-linking density, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
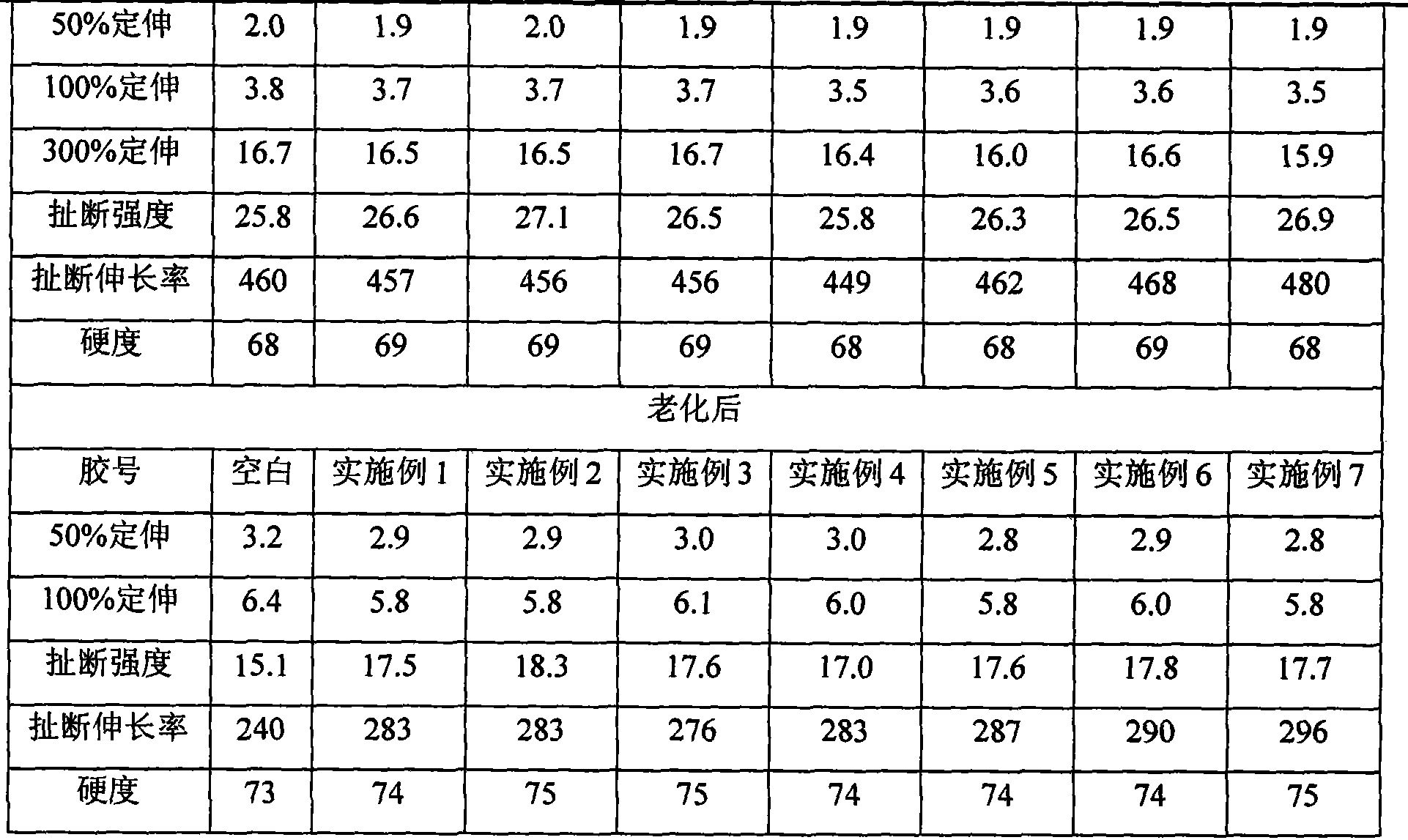
Examples
Embodiment 1
[0030] A 200mL four-necked flask equipped with a stirring device, a thermometer, and a reflux condenser was used as a reaction vessel, and 12.4g of sodium thiosulfate pentahydrate was weighed in the four-necked flask, and after adding 100mL of water and stirring to dissolve, the above solution was heated and heated to make the reaction The temperature of the solution was raised to 50°C, and 3.8 g of chloropropene was added dropwise while stirring, and at the same time, a metering pump was used to continuously add a sodium hydroxide solution with a concentration of 0.5 mol / L at a rate of 0.6 mL / h, so that the pH of the solution was 9.0 , after stirring and reacting under reflux for 6h, pour out the solution and add 100mL of ethanol, let the solution stand at 0°C for 1h, separate the solid matter, and dry it to constant weight at 55°C to obtain the bifunctional organic thiosulfate The chemical formula is CH 2 =CH-CH 2 -S 2 o 3 Na.
Embodiment 2
[0032]A 200mL four-necked flask equipped with a stirring device, a thermometer and a reflux condenser was used as a reaction vessel, and 18.6g of sodium thiosulfate pentahydrate was weighed in the four-necked flask, and after adding 100mL of water and stirring to dissolve, the above solution was heated up to make the reaction The temperature of the solution was raised to 50°C, and 5.7 g of chloropropene was added dropwise while stirring, and a sodium bicarbonate-sodium hydroxide buffer solution with a pH value of 9.6 was continuously added using a metering pump at a rate of 8.0 mL / h to make the pH of the solution After stirring and reacting for 6 hours under reflux, pour out the solution and add 300mL of ethanol, let the solution stand at 20°C for 6h, separate the solid product, dry it at 55°C to constant weight, and obtain the bifunctional organic thiosulfuric acid Salt chemical formula is CH 2 =CH-CH 2 -S 2 o 3 Na.
Embodiment 3
[0034] A 200mL four-necked flask equipped with a stirring device, a thermometer, and a reflux condenser was used as a reaction vessel, and 49.6g of sodium thiosulfate pentahydrate was weighed in the four-necked flask. The temperature of the solution was raised to 50°C, and 20 g of allyl chloride was added dropwise while stirring, and at the same time, a metering pump was used to continuously add sodium bicarbonate solution with a concentration of 0.5 mol / L at a rate of 2.0 mL / h, so that the pH of the solution was 7.5. After stirring and reacting for 9 hours under reflux, the solution was poured out and 600 mL of ethanol was added, the solution was left standing at 4°C for 6 hours, and the product was separated and dried at 50°C to constant weight to obtain the chemical formula CH 2 =CH-CH 2 -S 2 o 3 Bifunctional organothiosulfates of Na.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com