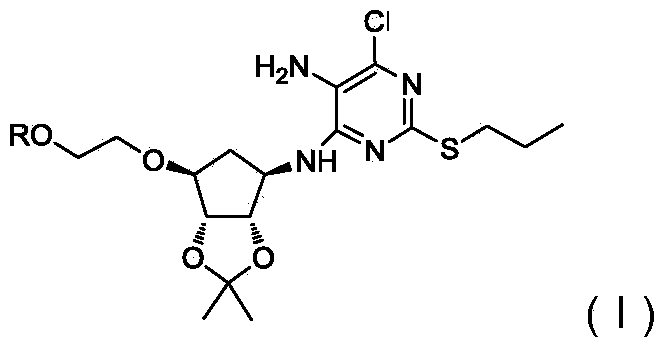
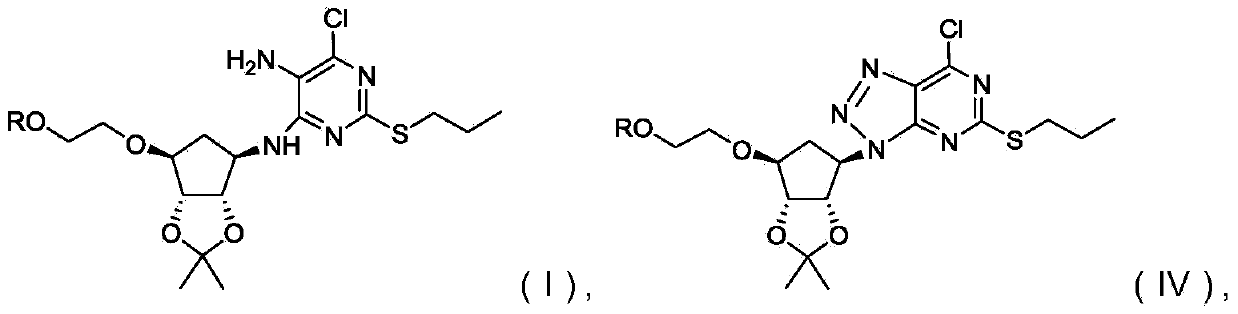
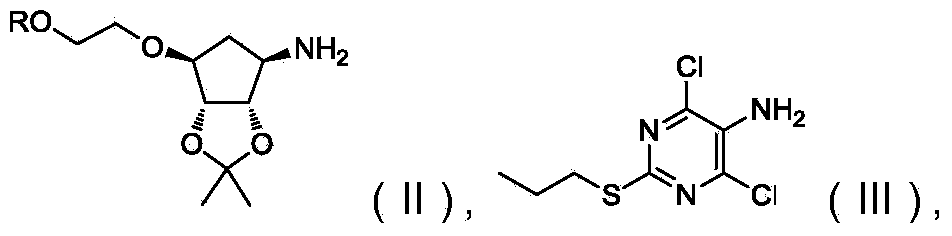Novel intermediate of ticagrelor and preparation method thereof
A technology of ticagrelor and a compound is applied in the field of novel intermediates of antiplatelet aggregation drug ticagrelor and its preparation, which can solve the problems of unfavorable industrialized production, lengthy process operation, long reaction time and the like, and achieves short reaction time and high purity. High, easy post-processing results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 2-[(3aR,4S,6R,6aS)-6-Benzyl carbamate-2,2-dimethyltetrahydro-3aH-cyclopentadieno[d][1,3]-dioxane Pent-4-ol
[0037]
[0038] Add (3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopentadieno[d][1,3]-dioxane to a 250ml reaction flask Pent-4-ol (3.0g), ethyl acetate 60ml, potassium carbonate (4.84g). Cool to 0°C, add 20ml of water, dissolve and clarify. Benzyl chloroformate (3.6 g) was added dropwise while maintaining 0°C. After the dropwise addition, keep warm for 20 minutes, rise to room temperature and stir for 3 hours, extract with ethyl acetate, and wash the organic layer once with 20 ml of water. The organic layer was concentrated under reduced pressure to obtain compound a: 2.68 g.
Embodiment 2
[0040] 2-{[(3aR,4S,6R,6aS)-6-Benzyl carbamate-2,2-dimethyltetrahydro-3aH-cyclopentadieno[d[1,3]-dioxane Pent-4-yl]oxy}-1-benzoic acid ethyl ester
[0041]
[0042] Compound a (2.68g) was dissolved in 30ml of tetrahydrofuran, then potassium tert-butoxide (1.5g) was added and cooled to -10°C. A tetrahydrofuran solution of ethyl 2-bromobenzoate (3.0 g) was slowly added, reacted at -10°C for 2 hours, and the organic phase was washed twice with 50 ml of water respectively. Concentrate through a silica gel column (EA:PE=1:6) to obtain compound b, 2.14g.
Embodiment 3
[0044] 2-{[(3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopentadieno[d[1,3]-dioxol-4 -yl]oxy}-1-ethyl benzoate
[0045]
[0046] Compound b (2.0 g) was dissolved in 25 ml of methanol, and then 0.22 g of palladium carbon was added, then hydrogen gas was introduced at room temperature and pressure, stirred overnight and concentrated by filtration to obtain compound c, 1.2 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com