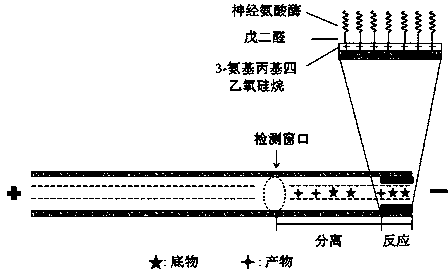
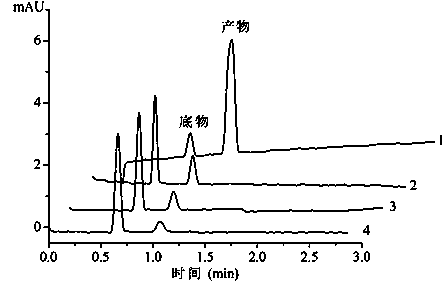
Preparation method of in-capillary immobilized enzyme microreactor
A microreactor, immobilized enzyme technology, applied in the direction of enzyme production/bioreactor, biochemical equipment and method, bioreactor/fermenter for specific purposes, etc., can solve the problem of poor reactor stability and easy washing of enzymes Take off and other problems to achieve the effect of convenient operation, shortening the effective length, enhancing stability and service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A quartz capillary with an inner diameter of 50 μm and a length of 35.5 cm was selected, and was first washed with 1 M NaOH for 2 h, and then washed with H 2 Wash with O for 30 min and pure methanol for 30 min; then fix the capillary on a capillary electrophoresis apparatus, inject a 30% by volume methanol solution of 3-aminopropyltetraethoxysilane from the outlet of the capillary with a pressure of 50 mbar 6 s, after standing at room temperature for 1 h, then rinse with pure methanol from the inlet end of the capillary for 5 min, H 2 Rinse with O for 3 min, pH7.0 Na 2 HPO 4 -NaH 2 PO 4 The buffer solution was washed for 5 min; then 2.5% glutaraldehyde solution was injected from the outlet end of the capillary with a pressure of 50 mbar for 6 s, and left at room temperature for 1 h, then Na 2 HPO 4 -NaH 2 PO 4 The buffer solution was washed for 5 min; finally, 0.1 U / mL neuraminidase solution was injected from the outlet end of the capillary with a pressure of 50 ...
Embodiment 2
[0035] A quartz capillary with an inner diameter of 50 μm and a tube length of 38.5 cm was selected, firstly rinsed with 1 M NaOH for 2 h, and then washed with H 2 Wash with O for 30 min and pure methanol for 30 min; then fix the capillary on a capillary electrophoresis apparatus, inject a 30% by volume methanol solution of 3-aminopropyltetraethoxysilane from the outlet of the capillary with a pressure of 50 mbar 12s, after standing at room temperature for 1 h, then rinse with pure methanol from the capillary inlet for 5 min, H 2 O rinse for 3 min, pH 7.0 Na 2 HPO 4 -NaH 2 PO 4The buffer solution was washed for 5 min; then, 3% glutaraldehyde solution was injected from the outlet end of the capillary with a pressure of 50 mbar for 12 seconds. 2 HPO 4 -NaH 2 PO 4 The buffer solution was washed for 5 min; finally, 0.1 U / mL neuraminidase solution was injected from the outlet end of the capillary with a pressure of 50 mbar for 12 s, left at room temperature for 30 min, and s...
Embodiment 3
[0038] A quartz capillary with an inner diameter of 50 μm and a length of 40.5 cm was selected, and was first washed with 1 M NaOH for 2 h, and then washed with H 2 Wash with O for 30 min and pure methanol for 30 min; then fix the capillary on a capillary electrophoresis apparatus, inject a 30% by volume methanol solution of 3-aminopropyltetraethoxysilane from the outlet of the capillary with a pressure of 50 mbar 18 s, after standing at room temperature for 2 h, then rinse with pure methanol from the inlet end of the capillary for 5 min, H 2 Rinse with O for 3 min, pH 7.0 Na 2 HPO 4 -NaH 2 PO 4 The buffer solution was washed for 5 min; then, 5% glutaraldehyde solution was injected from the outlet of the capillary with a pressure of 50 mbar for 18 s, left at room temperature for 3 h, and then injected with pH 7.0 Na 2 HPO 4 -NaH 2 PO 4 The buffer solution was washed for 5 min; finally, 0.1 U / mL neuraminidase solution was injected from the outlet end of the capillary wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com