Soyasapogenol composition
A technology of soyasapogenol and composition, which is applied in the field of soyasapogenol composition and achieves the effects of easy preparation, high bioavailability and high absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 2
[0115] 1. Preparation of Soybean Soap Alcohol Composition
[0116] 50g of soybean saponin glycoside (product name: saponin AZ-B, product of J-OIL MILLS, INC.) containing 23.2wt% A group saponin glycoside and 53.0wt% B group saponin glycoside was packed in a 5000ml glass container, Then this soybean saponin glycoside is dissolved in the ethanol of 1600ml80%. Concentrated sulfuric acid was added to the obtained solution so that the sulfuric acid concentration was 2N. The obtained reaction solution was kept at 70° C. to start the acid decomposition reaction of saponin glycoside.
[0117] After the reaction started, 150 mL of the reaction solution was collected every hour (a total of 8 hours) and neutralized with 1N NaOH. The cake obtained by centrifuging each neutralized product was washed with water, and then dried in a Yamato vacuum drying oven DP-301 (product of Yamato Scientific Co., Ltd.) to obtain a powder. The composition of Example 1 was prepared by mixing 1.07 g of th...
Embodiment 3 to 4
[0130] 1. Preparation of Soybean Soap Alcohol Composition
[0131] Except changing soybean saponin glycoside into saponin AZ-B (the product of J-OIL MILLS, INC.) containing 31.9wt% A group saponin glycoside and 60.0wt% B group saponin glycoside, in the same manner as in Example 1 Partial acid decomposition of soybean saponin glycosides in the same manner.
[0132] After the reaction started, 300 mL of the reaction solution was collected at 3 hours (Example 3) and 6 hours (Example 4) and neutralized with 1N NaOH solution. The cake obtained by centrifuging each neutralized product was dried in a Yamato vacuum drying oven DP-301 (product of Yamato Scientific Co., Ltd.) to obtain a powder. The analytical values for the compositions of Examples 3 and 4 are shown in Table 2.
[0133] [Table 2]
[0134]
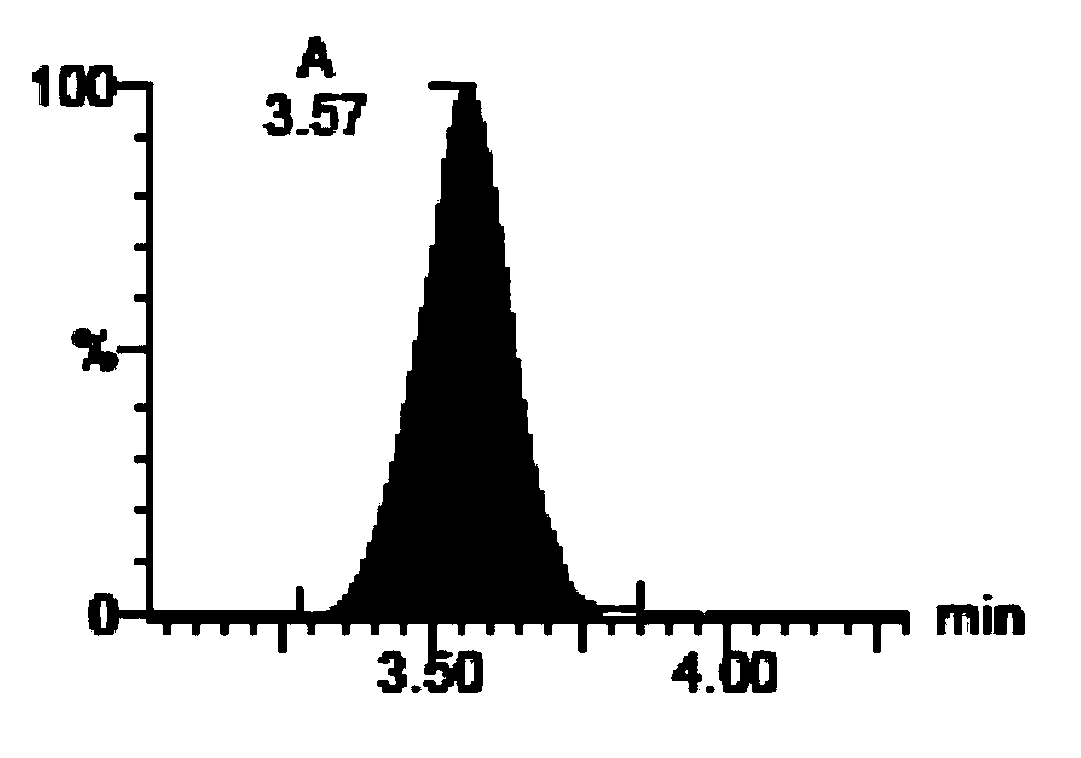
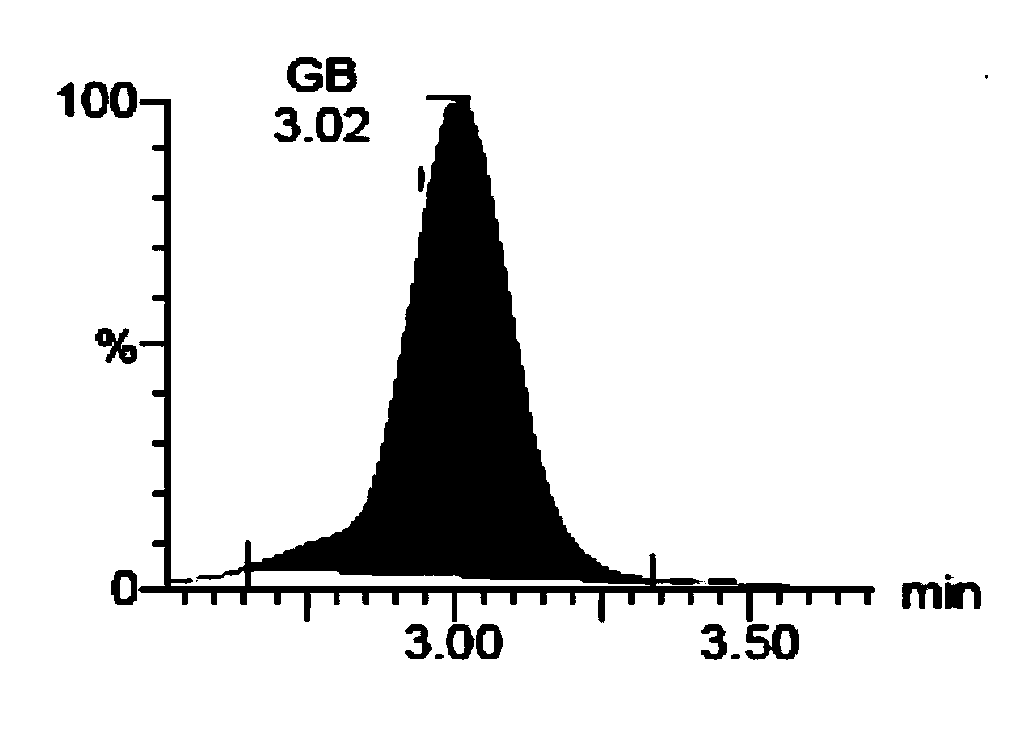
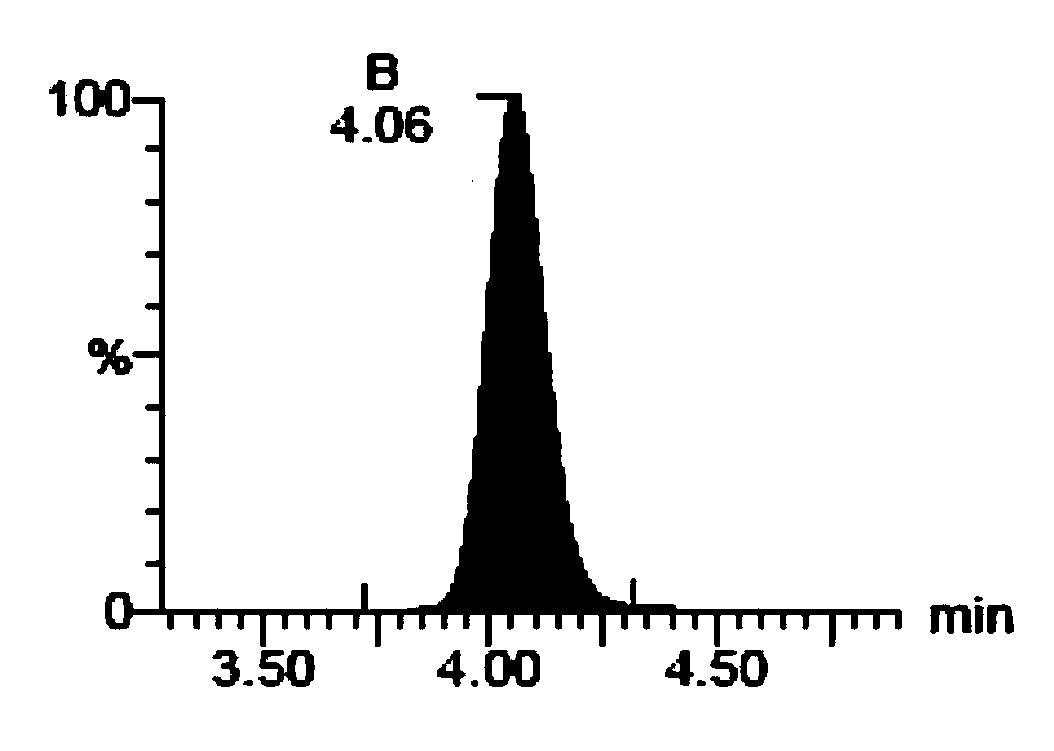
[0135] (A) Soy Saponol B
[0136] (B) 3-O-D-Glucopyranosyl Soy Saponol B
[0137] (C) Soy Saponol A
[0138] 2. Absorption evaluation of soybean saponol composition
[013...
Embodiment 5 to 10
[0144] 1. Preparation of Soybean Soap Alcohol Composition
[0145] The soybean saponin glycoside used in the embodiment 3 of 150g is packed in the glass container of 3000ml, and described glucoside is dissolved in the ethanol of 1500ml80%. Concentrated sulfuric acid was added to the obtained solution so that the sulfuric acid concentration was 2N. The solution was maintained at 80° C. for 72 hours with slow stirring to allow partial acid decomposition of the saponin glycosides.
[0146] 100 ml of the reaction solution was collected before and after the reaction, as shown in Table 3, and each collected reaction solution was neutralized with 1N NaOH solution. The neutralized product is dried by an evaporator to obtain a powder. In Table 3, the analytical values of the obtained compositions are shown.
[0147] 2. Absorption evaluation of soybean saponol composition
[0148] 5-week-old male SD rats (Charles River Laboratories Japan, Inc.) were divided into groups so that eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com