Citrazinic acid lead-zirconium double metal salts and preparation method and application thereof
A technology of lead citrazinate and bimetallic salt, which is applied in the field of lead zirconium citrazine bimetallic salt and its preparation and application, can solve the problem of high frequency oscillation instability, low melting point, and cannot meet the needs of new solid advancement and development, etc. problem, to achieve the effect of improving catalytic effect and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
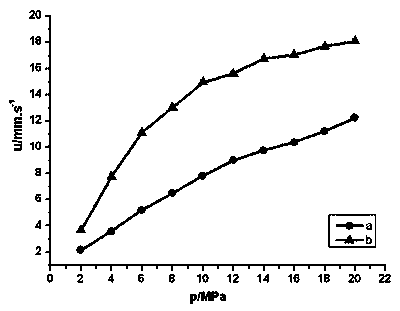
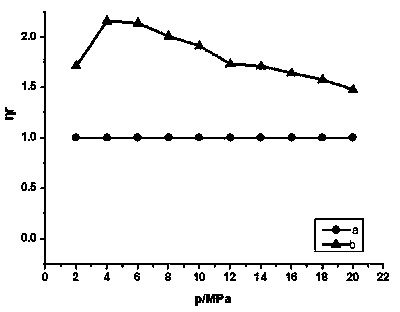
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of lead zirconium citracinate bimetallic salt
[0024] (1) Weigh 2.7g (0.01mol) of zirconyl nitrate and dissolve in 30mL of deionized water to prepare an aqueous solution of zirconyl nitrate; weigh 3.1g (0.02mol) of citrazine acid in 200mL of deionized water and add 1.7g (0.02mol) Sodium bicarbonate dissolved into a clear solution;
[0025] (2) Weigh 3.31g (0.01mol) of lead nitrate and dissolve it in 50mL deionized water to form a solution, add it to the sodium citrate solution, stir and mix uniformly, heat up to 50°C, and react for 2h;
[0026] (3) Weigh 1.6g (0.04mol) of sodium hydroxide and dissolve it in 20mL of deionized water, add it to the lead sodium citrate solution of step (2), react for 0.5h, and add the nitrate prepared in step (1) The zirconium aqueous solution was heated to 70°C to react for 3 hours, then cooled and left standing. The precipitate was washed with deionized water, filtered, and dried to obtain 5.91 g of black lead-zirconium c...
Embodiment 2
[0036] Example 2 Preparation of lead zirconium citracinate bimetallic salt
[0037] (1) Weigh 2.7g (0.01mol) of zirconyl nitrate dissolved in 30mL of deionized water to prepare an aqueous solution of zirconyl nitrate; weigh 3.1g (0.02mol) of citrazine acid in 200mL of deionized water and add 1.1g (0.01mol) Sodium carbonate is dissolved into a clear solution;
[0038] (2) Weigh 3.31g (0.01mol) of lead nitrate and dissolve it in 50mL deionized water to form a solution, add it to the sodium citrate solution, stir and mix uniformly, heat up to 60°C, and react for 2h;
[0039] (3) Weigh 2.3g (0.04mol) of potassium hydroxide and dissolve it in 20mL of deionized water, add it to the lead sodium citrate solution of step (2), react for 0.5h, and add the nitrate oxygen prepared in step (1) The zirconium aqueous solution was heated to 80°C to react for 4 hours, then cooled and left standing. The precipitate was washed with deionized water, filtered, and dried several times to obtain 5.82g of b...
Embodiment 3
[0040] Example 3 Preparation of lead zirconium citracinate bimetallic salt
[0041] (1) Weigh 2.7g (0.01mol) of zirconyl nitrate dissolved in 30mL of deionized water to prepare an aqueous solution of zirconyl nitrate; weigh 3.1g (0.02mol) of citrazine acid in 200mL of deionized water and add 1.1g (0.01mol) Sodium carbonate is dissolved into a clear solution;
[0042] (2) Weigh 3.32g (0.01mol) of lead nitrate and dissolve it in 50mL deionized water to form a solution, add it to the sodium citrate solution, stir and mix uniformly, heat up to 70°C, and react for 2h;
[0043] (3) Weigh 2.3g (0.04mol) of potassium hydroxide and dissolve it in 20mL of deionized water, add it to the lead sodium citrate solution of step (2), react for 0.5h, and add the nitrate oxygen prepared in step (1) The zirconium aqueous solution was heated to 70°C to react for 3 hours, then cooled and stood still. The precipitate was washed with deionized water, filtered, and dried to obtain 5.75g of black lead-zircon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com