Extraction method of chondroitin sulfate
A chondroitin sulfate and extraction method technology, which is applied in the field of biochemical medicaments to achieve the effects of shortening extraction time, simplifying extraction process and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Cartilage and pure water are mixed at a ratio of 1:1;
[0036] (2) Add alkaline protease at a ratio of 0.5% to cartilage, and enzymatically react for 2 hours at 55°C and pH9.5;
[0037] (3) Add keratinase at a ratio of 0.5% to cartilage, and enzymatically react at 55°C and pH 10.5 for 3 hours;
[0038] (4) Filter, take the filtrate, add ethanol solution to the filtrate, make the final concentration of ethanol reach 66%, and settle for 1 hour;
[0039] (5) Centrifuge the sedimentation liquid, the conditions of centrifugation are 6000rpm, 5min, take the precipitate, and dry at 60℃ to obtain chondroitin sulfate.
[0040] In Example 1, the obtained chondroitin sulfate analysis results are as follows:
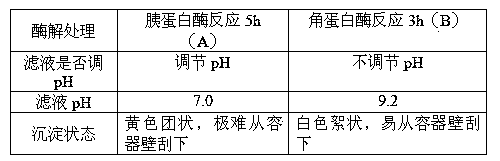
[0041] The obtained chondroitin sulfate 0.665mg / ml, the yield is 13.3%, white flocculent, easy to scrape from the container wall;
Embodiment 2
[0079] (1) Cartilage and pure water are mixed in a ratio of 1:2;
[0080] (2) Add alkaline protease according to the ratio of 0.60% to cartilage, and enzymatically react for 2.5h at 50℃ and pH9.6;
[0081] (3) Add keratinase at the ratio of 0.45% to cartilage, and enzymatically react for 2.5h at 60°C and pH 10.2;
[0082] (4) Filter, take the filtrate, add ethanol solution to the filtrate, make the final concentration of ethanol reach 69%, and settle for 1 hour;
[0083] (5) Centrifuge the sedimentation liquid. The conditions of the centrifugation are 6000rpm, 5min, take the precipitate and dry it at 60℃ to obtain chondroitin sulfate.
[0084] The method of the present invention has the advantages of short time and low cost, the content of chondroitin sulfate obtained by the method can reach 0.645 mg / ml, and the crude product yield is 12.9%.
Embodiment 3
[0086] (1) Cartilage and pure water are mixed in a ratio of 1:0.5;
[0087] (2) Add alkaline protease according to the ratio of 0.45% to cartilage, and enzymatically react for 1.5h at 60℃ and pH9.5;
[0088] (3) Add keratinase at a ratio of 0.55% to cartilage, and enzymatically react for 3.5 hours at 55°C and pH 10.5;
[0089] (4) Filter, take the filtrate, add ethanol solution to the filtrate, make the final concentration of ethanol reach 62%, and settle for 1 hour;
[0090] (5) Centrifuge the sedimentation liquid. The conditions of the centrifugation are 6000rpm, 5min, take the precipitate and dry it at 60°C to obtain chondroitin sulfate.
[0091] The method of the present invention has the advantages of short time and low cost, the content of chondroitin sulfate obtained by the method can reach 0.618 mg / ml, and the crude product yield is 12.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com