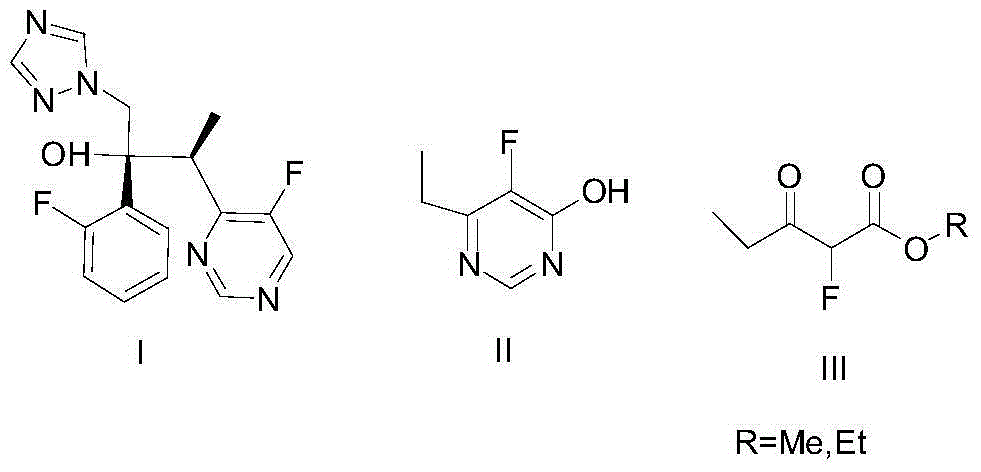
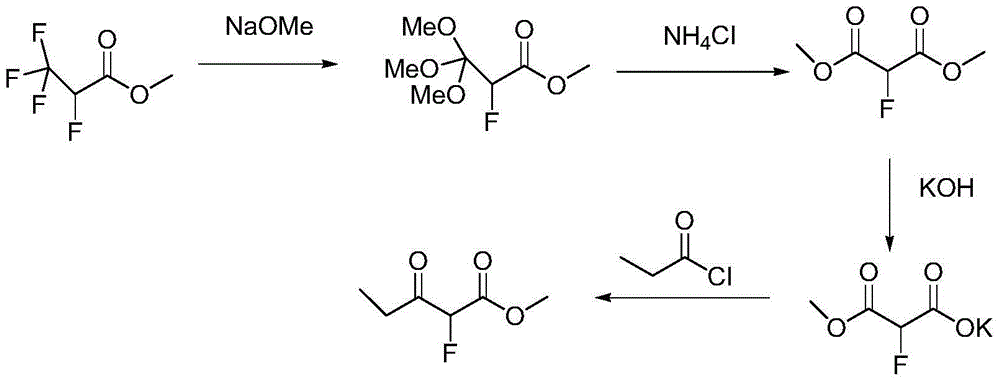
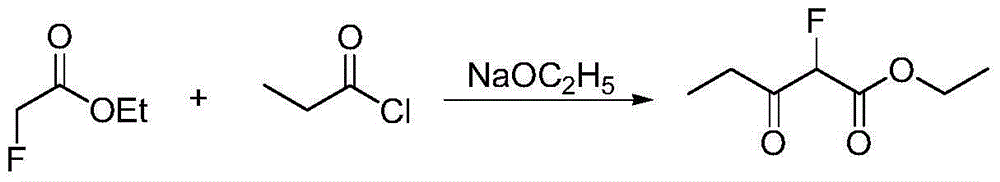Method for preparing a-fluoro-propionyl acetate
A technology of fluoropropionyl acetate and chloropropionyl acetate, which is applied in the field of synthesis of voriconazole intermediate a-fluoropropionyl acetate, which can solve unfavorable industrial production, high equipment requirements, and safety Low-level problems, to achieve the effect of safe production process, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 2L reaction flask, add 78.0g (0.599mol) of methyl propionoacetate and 500ml of dichloromethane, stir and dissolve at room temperature, slowly add 93.5g (0.693mol) of sulfonyl chloride dropwise, and stir at room temperature for 2 -3h, after the reaction, lower the temperature to 0-5°C, add 200ml of water and stir for 20min, extract the aqueous phase with 100ml of dichloromethane once, combine the organic phase, and use 10% sodium carbonate solution (200ml) and saturated saline for the organic phase in turn (200ml) was washed, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 96g of methyl α-chloropropionoacetate with a yield of 98%.
[0026] In a 1L reaction flask, add 90.0g (0.547mol) of methyl α-chloropropionoacetate, 300ml of DMF, 35.0g (0.602mol) of potassium fluoride, 2.25g of polyethylene glycol 600, stir under nitrogen protection, and heat Raise the temperature to 130°C, react for 10 hours, after the reaction is completed,...
Embodiment 2
[0028] In a 2L reaction flask, add 78.0g of ethyl propionoacetate and 500ml of n-hexane, stir and dissolve at room temperature, slowly add 93.5g of sulfonyl chloride dropwise, and stir at room temperature for 2-3h after the dropwise completion. After the reaction, cool down to 0-5°C, add 200ml of water and stir for 20min, extract the aqueous phase with 100ml of dichloromethane once, combine the organic phase with 200ml of 10% sodium carbonate solution, wash with 200ml of saturated saline, dry the organic phase with anhydrous sodium sulfate, and concentrate under reduced pressure 94.1 g of ethyl α-chloropropionoacetate was obtained, with a yield of 97.0%.
[0029] In a 1L reaction flask, add 90.0g (0.541mol) ethyl α-chloropropionoacetate, 350ml N,N-dimethylacetamide, 34.66g (0.595mol) potassium fluoride, 4.5g polyethylene glycol 600, stirred under the protection of nitrogen, heated to 130°C, reacted for 10h, after the reaction was completed, cooled to room temperature, filtered...
Embodiment 3
[0031] In a 1L reaction flask, sequentially add 90.0g (0.547mol) methyl α-chloropropionoacetate, 300mL DMF, 34.96g (0.602mol) potassium fluoride, 2.25g polyethylene glycol 800, stir under nitrogen protection, and heat Raise the temperature to 130°C and react for 10 hours. After the reaction is completed, cool to room temperature and filter. The filtrate is concentrated under reduced pressure. The residue is added to 200ml of water and stirred for 15min. (200mlx2), the organic phase was dried with anhydrous sodium sulfate, filtered, concentrated under reduced pressure to remove the solvent, and the residue was rectified under reduced pressure to obtain 73.17g methyl a-fluoropropionoacetate, light yellow liquid, 85-90°C / 10mmHg, yield 90.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com