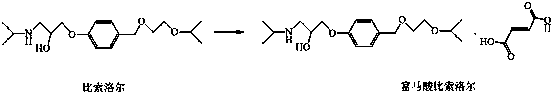New preparation method for bisoprolol fumarate
A technology of bisoprolol fumarate and sulfonic acid, which is applied in the preparation of carboxylate salts, organic compounds, aminohydroxyl compounds, etc. It can solve the problems of unavoidable intermolecular reactions, inability to recrystallize and purify, and many impurities. Problems, achieve high product purity, good purification and separation effect, and reduce impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
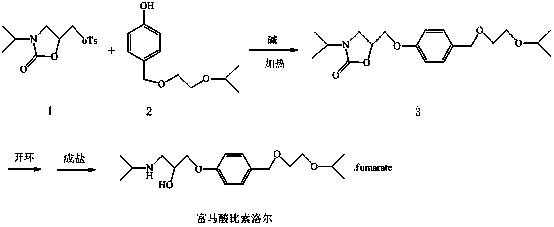
Embodiment 1
[0026] The synthesis of the middle 3 of embodiment 1
[0027]
[0028] the intermediate 1 (20g, 63.9mmol) was dissolved in 200ml of anhydrous N,N dimethylformamide, under the protection of argon, slowly added sodium hydride (1.7g, 70.3mmol), the intermediate 2 (14.8g, 70.3mmol), after addition, react at 25°C for 4h until complete reaction.
[0029] Post-processing: Evaporate N,N dimethylformamide to dryness under reduced pressure, add water / isopropyl ether for extraction, and wash the organic layer with 20% sodium hydroxide solution. The organic layer was dried and spin-dried to obtain 15.9 g of white semi-solid (intermediate 3 ), with a yield of 71%.
Embodiment 2
[0030] The synthesis of the middle 3 of embodiment 2
[0031] the intermediate 1 (20g, 63.9mmol) was dissolved in 200ml N,N dimethylformamide, under the protection of argon, slowly added sodium hydroxide (2.8g, 70.3mmol), the intermediate 2 (14.8g, 70.3mmol), after adding, react at 60°C for 4h until complete reaction.
[0032] Post-processing: Evaporate N,N dimethylformamide to dryness under reduced pressure, add water / isopropyl ether for extraction, and wash the organic layer with 20% sodium hydroxide solution. The organic layer was dried and spin-dried to obtain 13.1 g of white semi-solid (intermediate 3 ), with a yield of 58%.
Embodiment 3
[0033] The synthesis of the middle 3 of embodiment 3
[0034]
[0035] the intermediate 1 (20g, 63.9mmol) was dissolved in 200ml of anhydrous N,N dimethylformamide, under the protection of argon, slowly added potassium tert-butoxide (7.8g, 70.3mmol), the intermediate 2 (14.8g, 70.3mmol), after adding, react at 25°C for 5h until complete reaction.
[0036] Post-processing: Evaporate N,N dimethylformamide to dryness under reduced pressure, add water / isopropyl ether for extraction, and wash the organic layer with 20% sodium hydroxide solution. The organic layer was dried and spin-dried to obtain 13.8 g of white semi-solid (intermediate 3 ), with a yield of 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com