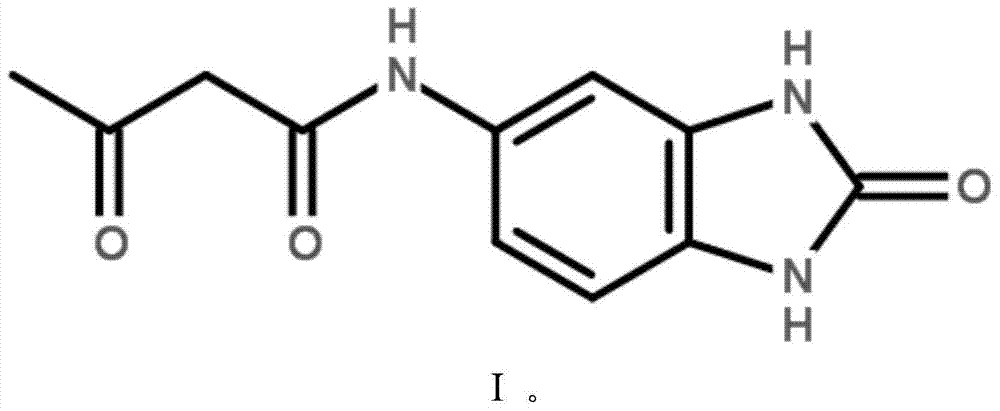
Preparation method for 5-acetoacetlamino benzimdazolone
A technology of acetoacetamidobenzimidazolone and aminobenzimidazolone is applied in the field of preparation of 5-acetoacetamidobenzimidazolone, and can solve the problem of low production level and quantity of 5-acetoacetamidobenzimidazolone. The quality cannot meet the growing needs, the lack of high-performance dyes and pigments, etc., to achieve the effect of reducing the risk factor, reducing equipment requirements, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a kind of preparation method of 5-acetoacetylamino benzimidazolone, comprising the following steps:
[0028] Under the action of an acid catalyst, react 5-aminobenzimidazolone, diketene, alcohol and water to obtain 5-acetoacetamidobenzimidazolone;
[0029] The alcohol is one or more of methanol, ethanol and propanol.
[0030] The preparation method provided by the present invention uses an acidic catalyst, and uses alcohol water as a solvent, which increases the solubility of 5-aminophenyl imidazolone, thereby promoting the acetylation reaction and reducing the reaction temperature, so that the reaction itself will not generate pressure. There is no need to continuously release pressure during the reaction process, thereby reducing the requirements for equipment, and also reducing the risk factor in actual production, making the reaction conditions mild and easy to operate.
[0031] All the raw materials of the present invention have no special l...
Embodiment 1
[0048] First, under the protection of nitrogen, 400 kg of o-phenylenediamine, 400 kg of urea, 3.7 kg of benzyltriethylammonium chloride, and 1000 kg of chlorobenzene were added to the reactor, and the temperature was raised to 115°C for reaction. After 8 hours of reaction, To obtain the benzimidazolone solution, slowly add 260 kg of concentrated nitric acid with a mass content of 62% to it, react at 80°C for 3 hours, then cool down to 50°C, and distill p-chlorine under reduced pressure under 0.04Mpa pressure Benzene is recovered. After the recovery is completed, 2000 kg of deionized water is added to the reactor, boiled at a temperature of 80° C. for 3 hours for purification, and finally the obtained material is dried to obtain 5-nitrobenzimidazolone.
[0049] Then 200 kilograms of 5-nitrobenzimidazolone obtained by the above steps, 60 kilograms of hydrochloric acid with a mass content of 30%, and 180 kilograms of iron powder with a mass content of 75% are mixed with 4000 kilog...
Embodiment 2
[0054] First, under the protection of nitrogen, 400 kg of o-phenylenediamine, 400 kg of urea, 3.2 kg of tetrabutylammonium bromide, and 1000 kg of chlorobenzene were added to the reactor, and the temperature was raised to 115°C for reaction. After 8 hours of reaction, benzene was obtained. and imidazolone solution, and then slowly add 255 kg of concentrated nitric acid with a mass content of 62%, react at 80°C for 3 hours, then cool down to 50°C, and distill p-chlorobenzene under reduced pressure under 0.04Mpa pressure Recovery, after the recovery is completed, add 2000 kg of deionized water to the reaction kettle, boil in water for 3 hours at a temperature of 80° C., and perform purification treatment. Finally, the obtained material is dried to obtain 5-nitrobenzimidazolone.
[0055] Then 190 kilograms of 5-nitrobenzimidazolone obtained by the above steps, 58 kilograms of hydrochloric acid with a mass content of 30%, and 175 kilograms of iron powder with a mass content of 75% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com