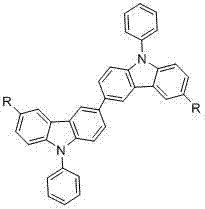
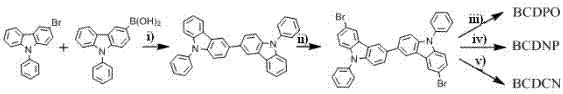
Dicarbazolyl derivative, preparation method and application of dicarbazolyl derivative, and electroluminescent device
An electroluminescent device, dicarbazole-based technology, applied in the field of dicarbazolyl derivatives and their preparation, electroluminescent devices, can solve the problems of limited application of blue phosphorescence host materials and weak electron transport ability, etc. Achieve the effect of improving the luminous efficiency of the device and reducing the efficiency roll-off
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
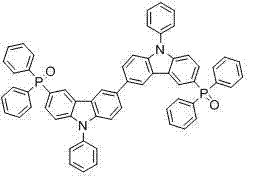
[0047] When R= , the compound is 3,3′-diphenylphosphoryloxy-6,6′ - Bis(9-phenyl)carbazole (BCDPO), the molecular structure is as follows image 3 shown.
[0048] Its preparation method comprises the following steps:
[0049] 3,3' - Bis(9-phenyl)carbazole (1.0 mmol), N -Bromosuccinimide (NBS) (2.05 mmol), silica gel (100 g) were dissolved in chloroform (150 ml), stirred at room temperature with tin foil in the dark for 24 hours, filtered directly with a sand core funnel, evaporated Dry organic solvent to get into 3,3' - Dibromo-bis(9-phenyl)carbazole is a white solid powder in 75% yield. MS (APCI): calcd for C 36 h 22 N 2 Br 2 : 642.0, found, 643.4 (M+1) + .
[0050] In a dry round bottom flask, sequentially add 3,3' - Dibromo-bis(9-phenyl)carbazole (1.0 mmol), diphenylphosphine oxide (2.0 mmol), nickel dichloride hexahydrate (NiCl 2 ·6H 2 O) (0.3 mmol), zinc powder (6.0 mmol), 2,2′-bipyridine (0.6 mmol), N,N' -Dimethylacetamide (DMAc) (30.0 ml), stirre...
Embodiment 2
[0052] When R 1 = , the compound is 3,3′-bis(2,4-pyrimidinyl)-6,6′ - Bis(9-phenyl)carbazole (BCDNP), the molecular structure is as follows Figure 4 shown.
[0053] Its preparation method comprises the following steps:
[0054] 3,3' - Bis(9-phenyl)carbazole (1.0 mmol), N -Bromosuccinimide (NBS) (2.05 mmol), silica gel (100 g) was dissolved in chloroform (150 ml), stirred at room temperature with tin foil in the dark for 24 hours, filtered directly with a sand core funnel, evaporated Dry organic solvent to get 3,3' - Dibromo-bis(9-phenyl)carbazole is a white solid powder in 75% yield. MS (APCI): calcd for C 36 h 22 N 2 Br 2 : 642.0, found, 643.4 (M+1) + .
[0055] In a dry round bottom flask, sequentially add 3,3' - Dibromo-bis(9-phenyl)carbazole (1.0 mmol), 2,4-pyrimidineboronic acid (2.1 mmol), Pd(PPh3 ) 4 (0.08 mmol), K 2 CO 3 (2M) (8 ml) toluene (50 ml), ethanol (20 ml), under nitrogen protection conditions, stirred at 110 °C for 24 hours, after the reac...
Embodiment 3
[0057] When R= , the compound is 3,3′-dicyano-6,6′ - Bis(9-phenyl)carbazole (BCDCN); Molecular structural formula such as Figure 5 shown.
[0058] Its preparation method comprises the following steps:
[0059] 3,3' - Bis(9-phenyl)carbazole (1.0 mmol), N -Bromosuccinimide (NBS) (2.05 mmol), silica gel (100 g) was dissolved in chloroform (150 ml), stirred at room temperature with tin foil in the dark for 24 hours, filtered directly with a sand core funnel, evaporated Dry organic solvent to get 3,3' - Dibromo-bis(9-phenyl)carbazole is a white solid powder in 75% yield. MS (APCI): calcd for C 36 H 22 N 2 Br 2 : 642.0, found, 643.4 (M+1) + .
[0060] 3,3' - Dibromo-bis(9-phenyl)carbazole ( 2 ) (1.0 mmol), CuCN (3.0 mmol), anhydrous DMF (50 ml), added to a round bottom flask, heated to reflux under nitrogen atmosphere for 24 hours, cooled to room temperature, added FeCl dropwise 3 Hydrochloric acid solution, the mixture was stirred at 70°C for 30 minutes, the organi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com