Porcine epidemic diarrhea virus resistant hyper-immune serum and preparation method thereof
A porcine epidemic diarrhea and hyperimmune serum technology is applied in antiviral agents, pharmaceutical formulas, antibody medical components, etc. It can solve the problems of weakened hyperimmune serum treatment effect, increased morbidity and mortality of infected pigs, etc., and achieves physical properties Good, high antibody titer, high cure rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of Porcine Epidemic Diarrhea Virus ZJ08 Strain Immunogen
[0022] Take an appropriate amount of porcine epidemic diarrhea-positive small intestine from a pig farm in Zhejiang, scrape the intestinal mucosa and contents, add PBS at a ratio of 1:5 (weight:volume), freeze and thaw repeatedly 3 times, centrifuge to take the supernatant, and filter it with a 0.22 μm membrane After filtration, trypsin with a final concentration of 20 μg / ml was added to the filtrate, and treated at 37° C. for 1.5 hours.
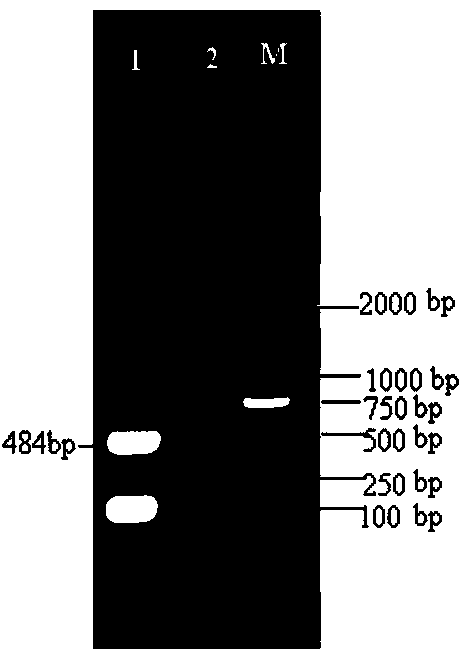
[0023] Inoculate monolayer Vero cells according to the conventional method, inoculate the virus at a ratio of 10%, absorb at 37°C for 1 hour, supplement the cell maintenance solution (containing 10 μg / ml trypsin), and incubate in a 37°C incubator. When passed to the 17th generation, obvious and stable CPE changes appeared, the cells shrank, the granules increased, and the piles were like bunches of grapes, and the cells were damaged and shed ( figure 1 )...
Embodiment 2
[0038] Example 2 Serum Preparation
[0039] 1) Immunization program
[0040] The first immunization: a total of 4ml intramuscular injection.
[0041] The second immunization: 14 days after the first immunization, the immunization dose is 8ml, and the injection is divided into two points.
[0042] The third immunization: 14 days after the second immunization, the immunization dose is 16ml, and injected in three points.
[0043] 2) blood collection
[0044]15-21 days after the last immunization, (the blood was collected once, the serum titer was measured by the neutralization test method, and then bled to death, with an interval of 3-4 days), the pigs were checked for health. No feed was given for the first 12 hours, but drinking water was not restricted. The butcher's knife and the skin where the knife enters are disinfected in advance, and the shallow basin containing blood is cleaned and then steam-sterilized. Sterile bloodletting.
[0045] 3) Serum separation and prepa...
Embodiment 3
[0048] Example 3 Determination of serum titer
[0049] Dilute the tested hyperimmune serum with DMEM at 1:2, 1:16, 1:32...1:512, and add an equivalent titer of 200 TCID to each dilution serum 50 / 0.1ml PEDV ZJ08 strain, neutralize at 37°C for 1 hour, inoculate 6 wells of a 96-well cell plate covered with Vero cell monolayer, 100 μl per well, and set 6 wells of non-neutralizing virus positive control cells and only inoculate cells Maintenance solution Negative control cells 6 wells, 37°C 5% CO 2 Cultivate in an incubator for 3 to 5 days, and observe CPE every day. There should be no cytopathic changes in the cell wells of the neutralization group and the negative control group, but the cells of the virus control group should appear cytopathic. The neutralization titer of the serum was taken as the highest dilution of the serum that could protect 50% of the cells from pathological changes. As can be seen from Table 1, the prepared anti-porcine epidemic diarrhea virus hyperimmu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com