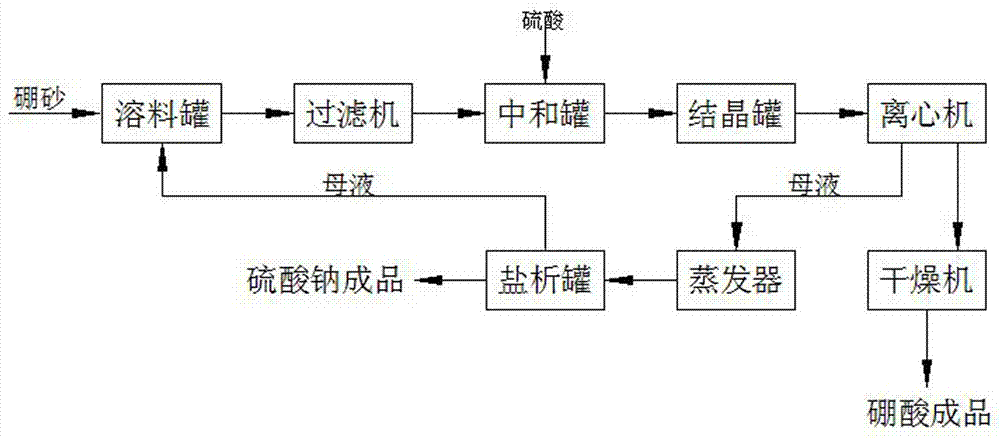
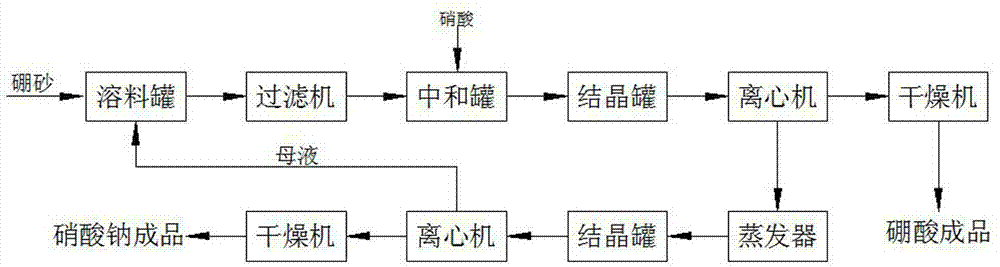
Method for preparing boric acid and sodium nitrate by processing native borax with nitric acid
A technology of sodium nitrate and borax ore, which is applied in the preparation of alkali metal nitrates and boron oxides, etc., can solve the problems of high energy consumption, achieve the effects of reducing energy consumption, reducing raw material costs, and saving equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
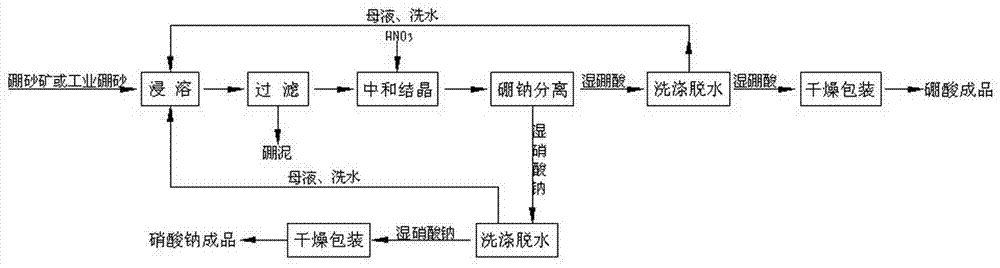
[0039] Such as image 3 Shown the present invention prepares the method for boric acid and sodium nitrate with nitric acid treatment borax mine, prepares by following steps:
[0040] Mix 1000kg of borax ore (containing 77% of borax decahydrate) and 5000L of boric acid mother liquor (co-saturated solution of boric acid and sodium nitrate at 20°C) in a immersion tank, heat to 90°C under stirring, and then time Heat preservation and leaching for 30 minutes. After leaching, pump the ore slurry into a filter press for filtration to obtain borax solution and boron mud. The borax solution is filtered twice, and the borax solution filtered twice is sent to the neutralization reactor. The temperature of the borax solution is controlled at 60°C, and 600L of nitric acid with a concentration of 45% is added under stirring conditions to control the end point of the neutralization reaction. The pH value of the reaction is 1.5, and then the neutralized liquid is sent to a cooling crystalliz...
Embodiment 2
[0043] Such as image 3 Shown the present invention prepares the method for boric acid and sodium nitrate with nitric acid treatment borax mine, prepares by following steps:
[0044] Mix 1000kg borax ore (containing 60% borax decahydrate) and 5500L boric acid mother liquor (co-saturated solution of boric acid and sodium nitrate at 23°C) in a immersion tank, heat to 93°C under stirring, and then time Heat preservation and leaching for 50 minutes. After leaching, pump the ore slurry into a filter press to filter to obtain borax solution and boron mud. Filter the borax solution twice, and send the second-filtered borax solution into the neutralization reactor, control the temperature of the borax solution at 70°C, add 280L of nitric acid with a concentration of 60% under stirring conditions, and control the end point of the neutralization reaction The pH value of the reaction is 3.5, and then the neutralized liquid is sent to a cooling crystallizer for crystallization. The crys...
Embodiment 3
[0047] Such as image 3 Shown the present invention prepares the method for boric acid and sodium nitrate with nitric acid treatment borax mine, prepares by following steps:
[0048] Mix 1000kg borax ore (containing 57% borax decahydrate) and 6000L boric acid mother liquor (co-saturated solution of boric acid and sodium nitrate at 25°C) in a immersion tank, heat to 95°C under stirring, and then time Heat preservation and leaching for 60 minutes. After leaching, pump the ore slurry into a filter press to filter to obtain borax solution and boron mud. Filter the borax solution twice, and send the second-filtered borax solution into the neutralization reactor, control the temperature of the borax solution at 85°C, add 290L of nitric acid with a concentration of 65% under stirring conditions, and control the end point of the neutralization reaction The pH value of the reaction is 2.0, and then the neutralized liquid is sent to a cooling crystallizer for crystallization. The crys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com