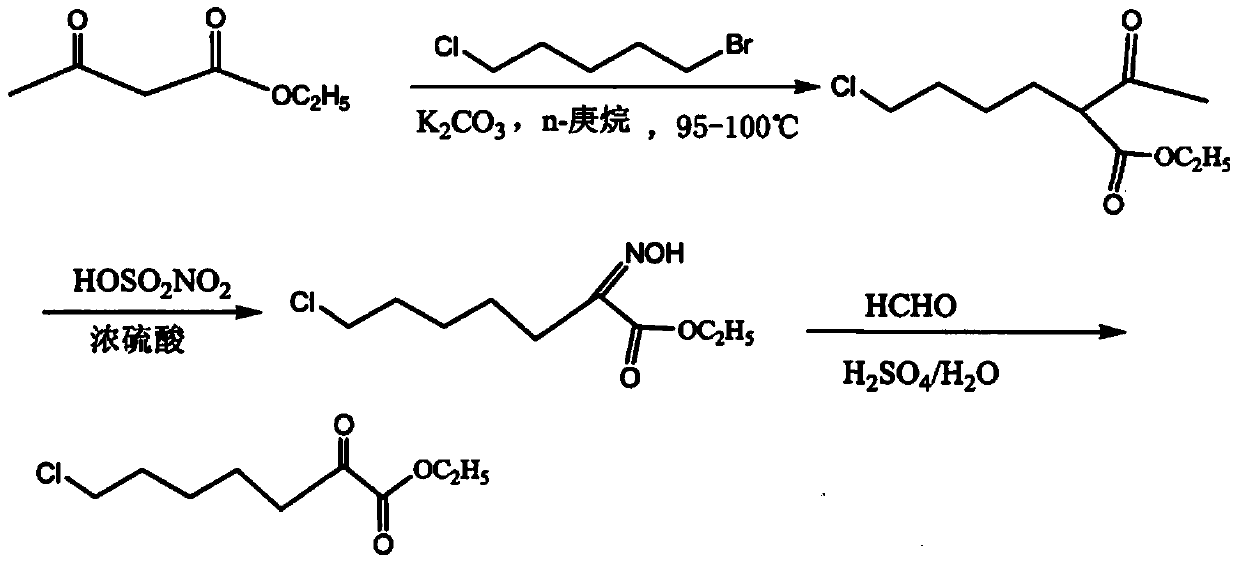
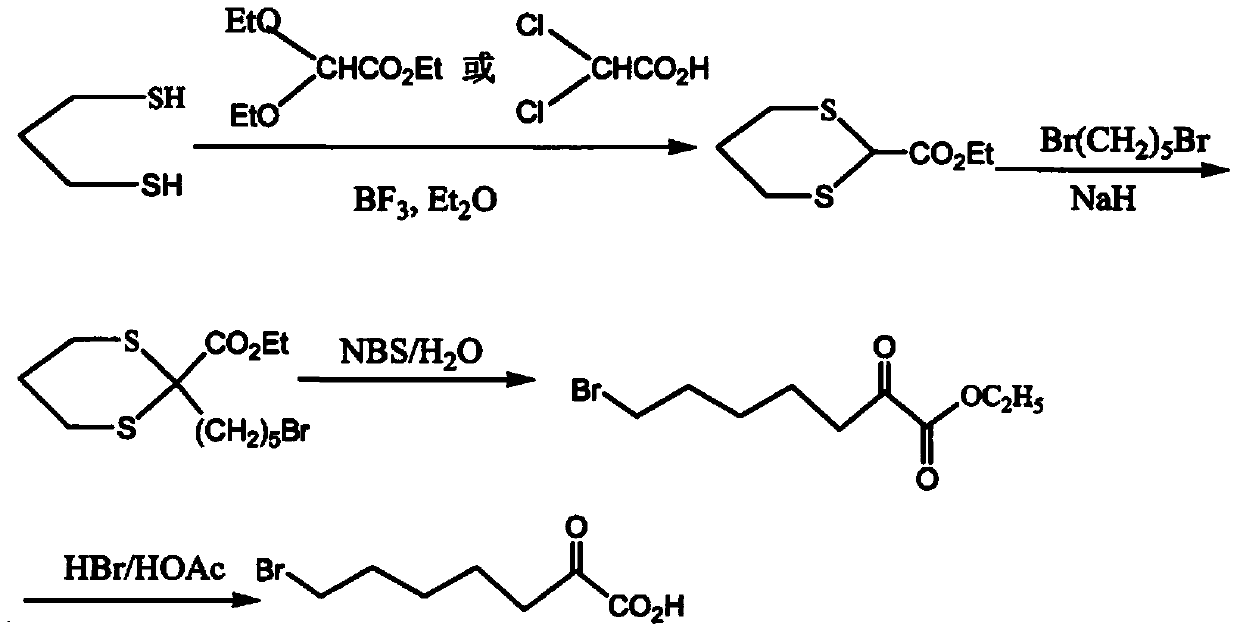
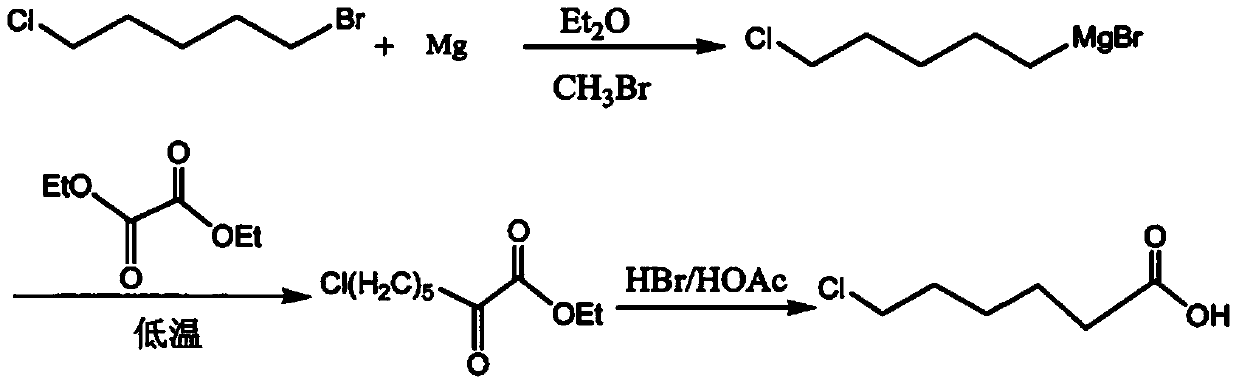
Preparation method of 7-chloro-2-oxoheptanoic acid ethyl ester
A technology of ethyl oxoheptanoate and monoethyl oxalate chloride, applied in the field of preparation of ethyl 7-chloro-2-oxoheptanoate, can solve the problem of low reaction yield, 7-chloro-2-oxo Ethyl heptanoate product has low purity and difficulty in preparing Grignard reagent, so as to achieve the effect of reducing production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] According to the preparation method of this example, using 1-bromo-5-chloropentane as the starting material, 7-chloro-2-oxoheptanoic acid ethyl ester was prepared through the following steps:
[0050](1) Add 15g (0.625mol) of magnesium into a 500ml four-neck flask, add 20ml of anhydrous THF and 1ml of 1-bromo-5-chloropentane and stir to initiate the reaction. 100g (0.54mol) of 1-bromo-5-chloropentane was mixed with 400ml of anhydrous THF, and then added dropwise to the four-necked flask. During the dropwise addition, the temperature of the material in the four-necked flask was kept at -10°C to -15°C After dropping, react until the magnesium powder completely disappears to obtain Grignard solution, detect 1-bromo-5-chloropentane residual ≤ 1%, seal it for use;
[0051] (2) In another dry four-neck flask, under mechanical stirring and nitrogen protection, add THF100ml and oxalic acid monoethyl chloride 85g (0.62mol), cool down to -15℃~-10℃, add dropwise 1) For the above-...
Embodiment 2
[0054] According to the preparation method of this example, using 1-bromo-5-chloropentane as the starting material, 7-chloro-2-oxoheptanoic acid ethyl ester was prepared through the following steps:
[0055] (1) Add 24g (1mol) of magnesium into a 500ml four-neck flask, add 20ml of anhydrous THF and 1ml of 1-bromo-5-chloropentane and stir to initiate the reaction. Mix 168g (0.9mol) of 1-bromo-5-chloropentane with 600ml of methyl tetrahydrofuran and add it dropwise into a four-necked flask. During the dropwise addition, the temperature of the material in the four-necked flask is kept at -10°C to -15°C After dropping, react until the magnesium powder completely disappears to obtain Grignard solution, detect 1-bromo-5-chloropentane residual ≤ 1%, seal it for use;
[0056] (2) In a dry 1L four-neck flask, under mechanical stirring and nitrogen protection, add 200ml of methyl tetrahydrofuran and 150g (1.1mol) of monoethyl oxalate chloride, cool down to -10°C ~ -15°C, dropwise add F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com