Preparation method of cortisone acetate
A technology of cortisone acetate and glacial acetic acid, applied in the direction of steroids, organic chemistry, etc., can solve the problems of insufficient quality and yield, increased production cost, and many side reactions, so as to achieve good product quality and yield, save Production costs, effects of avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] A preparation method of cortisone acetate, using 11α-hydroxyl-16α, 17α-epoxy progesterone as raw material, comprising the following steps:
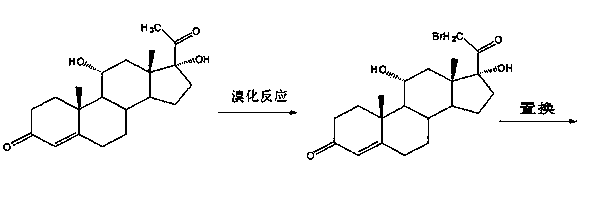
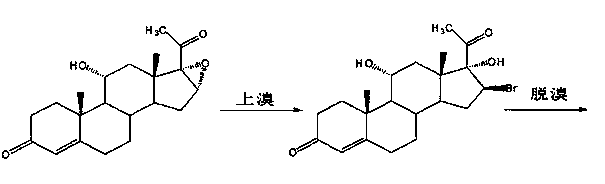
[0022] (a) Upper bromine reaction:
[0023] Add 11α-hydroxyl-16α, 17α-epoxyprogesterone into hydrobromic acid, stir and react at 5~10°C for more than 5 hours, after the reaction is completed, add water for water analysis, filter, wash with water until neutral, and dry to obtain 11α, 17α-dihydroxy-16β-bromoprogesterone;
[0024] (b) debromination reaction:
[0025] Add 11α,17α-dihydroxy-16β-bromoprogesterone into ethanol and raise the temperature to 40-45°C and stir to dissolve, then add glacial acetic acid, active Raney nickel and pass hydrogen in sequence, keep the reaction for 3 hours, filter, concentrate and recover under reduced pressure solvent, filtered, washed with water until neutral, and dried to obtain 11α, 17α-dihydroxyprogesterone;
[0026] (c) Bromination reaction:
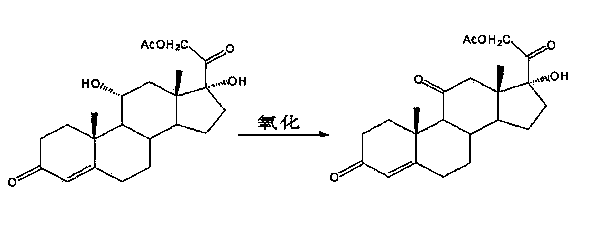
[0027] Add 11α, 17α-dihydroxyprogesterone into...
Embodiment 1
[0038] A preparation method of cortisone acetate, comprising the steps of:
[0039] (a) Bromine reaction: Preparation of 11α,17α-dihydroxy-16β-bromoprogesterone
[0040] Put 50g of 11α-hydroxyl-16α,17α-epoxyprogesterone and 150ml of hydrobromic acid into the reaction flask, stir and react at 5~10°C for 5 hours; after the reaction is completed, add 1000ml of water for water analysis, filter, and wash with water until neutral , drained, and dried to obtain 60 g of 11α, 17α-dihydroxy-16β-bromoprogesterone, with a TLC maximum point of 0.7%;
[0041] (b) Debromination reaction: Preparation of 11α,17α-dihydroxyprogesterone
[0042] Put 50g of 11α, 17α-dihydroxy-16β-bromoprogesterone and 1000ml of ethanol into the reaction bottle, heat up to 40-45℃ and stir to dissolve, then add 40ml of glacial acetic acid, 25g of Raney nickel in turn and pass hydrogen, keep the temperature for 3 hours , filtered, concentrated under reduced pressure to recover ethanol, filtered water to neutrality, s...
Embodiment 2
[0050] A preparation method of cortisone acetate, comprising the steps of:
[0051] (a) Bromine reaction: Preparation of 11α,17α-dihydroxy-16β-bromoprogesterone
[0052] Put 50g of 11α-hydroxyl-16α,17α-epoxyprogesterone and 150ml of hydrobromic acid into the reaction flask, stir and react at 5~10°C for 5 hours; after the reaction is completed, add 1000ml of water for water analysis, filter, and wash with water until neutral , drained, and dried to obtain 60 g of 11α, 17α-dihydroxy-16β-bromoprogesterone, with a TLC maximum point of 0.7%;
[0053] (b) Debromination reaction: Preparation of 11α,17α-dihydroxyprogesterone
[0054] Put 50g of 11α, 17α-dihydroxy-16β-bromoprogesterone and 1000ml of ethanol into the reaction bottle, heat up to 40-45℃ and stir to dissolve, then add 40ml of glacial acetic acid, 25g of Raney nickel in turn and pass hydrogen, keep the temperature for 3 hours , filtered, concentrated under reduced pressure to recover ethanol, filtered water to neutrality,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com