Type A foot-and-mouth disease CTL (cytotoxic T lymphocyte) epitope peptide and screening method thereof
A technology of epitope peptide and foot-and-mouth disease, applied in the field of molecular immunology, to achieve a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
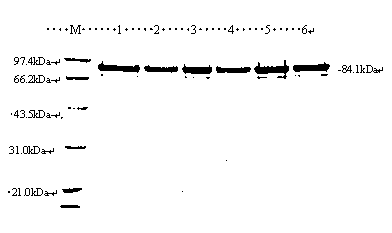
[0029] Example 1 Preparation of SAL-Ⅰ Protein from Six Strains of Pigs
[0030] (1) Preparation of six strains of porcine SLA-I / pMAL-p2X recombinant Escherichia coli
[0031] Six strains of pig SLA-I / pMAL-p2X recombinant Escherichia coli were constructed and preserved in the Laboratory of Molecular Immunology, School of Life Science and Technology, Dalian University. The six strains of pigs include Landrace, Yorkshire, Topek, Hebao, Yantai and Laiwu black pigs. The SLA-I / pMAL-p2X recombinant Escherichia coli was obtained by the method described in Gao Fengshan et al. (Gene, 2012, 502 (2): 147-153).
[0032] (2) Induced expression of SLA-I recombinant Escherichia coli
[0033] Inoculate 5 mL of the SLA-I / pMAL-p2X recombinant Escherichia coli liquid of six strains of pigs into 500 mL of LB medium, shake and culture in a 37-degree incubator at 170 rpm, and detect the OD at intervals 600 , to be grown to OD 600 When it is between 0.6 and 0.8, add IPTG to 0.5mmol / L, and indu...
Embodiment 2
[0041] Embodiment 2 Design of foot-and-mouth disease virus mimic epitope peptide
[0042] Using the biological information software netMHCpan2.4 (http: / / www.cbs.dtu.dk / services / NetMHCpan), input the O-type foot-and-mouth disease virus VP1 amino acid sequence (AJ539138), the A-type foot-and-mouth disease virus VP1 amino acid sequence (GQ406249) and Asia1 Type FMDV VP1 amino acid sequence (EF149009), select nonapeptide, and predict the CTL mimetic epitope peptide that can bind to SLA-?? protein under the SLA gene and present to the cell surface to cause cytotoxic immune response. Three 9-peptides derived from the VP1 protein of foot-and-mouth disease virus were named Q02, AS3 and QA4 respectively, and their amino acid sequences and other basic information are shown in Table 1. Among them, Q02 is derived from Tibet / CHA / 99, an epidemic strain of type O foot-and-mouth disease at home and abroad; AS3 is derived from the epidemic strain Asia 1 / Jiangsu / China / 2005 of type A foot-and-mo...
Embodiment 3
[0044] Example 3 The combination and screening of SLA-I protein and foot-and-mouth disease virus mimic epitope peptide
[0045] Mix 1 mL of each of the six strains of pig SLA-I proteins purified in Example 1 (protein content 1 mg / mL) with 5 mL of PBS solution, centrifuge at 4500 r / min for 20 min, remove the filtrate, add PBS to a total volume of about 5 mL, and repeat centrifugation Three times, take the upper PBS solution (containing SLA-I) and mix it with the peptide solution (Q02, AS3, QA4 or Co) respectively, the molar ratio of peptide to target protein is 10:1, overnight at 37°C. The mixture was added to a 30K ultrafiltration tube and centrifuged at 4500r / min for 45min. Add PBS, 37°C water bath for 2min, centrifuge at 4500r / min, until the final volume is about 100uL. Add 5mL of citric acid-phosphate buffer solution to the mixture and let it stand at room temperature for 2min. Transfer to a 5K ultrafiltration tube, centrifuge at 4500r / min for 5min, and collect about 5mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com