A kit, a system and a method for determining patient marrow microenvironment after hematopoietic stem cell transplantation
A technology of hematopoietic stem cells and kits, applied in measuring devices, instruments, fluorescence/phosphorescence, etc., can solve the problems of limited popularization and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
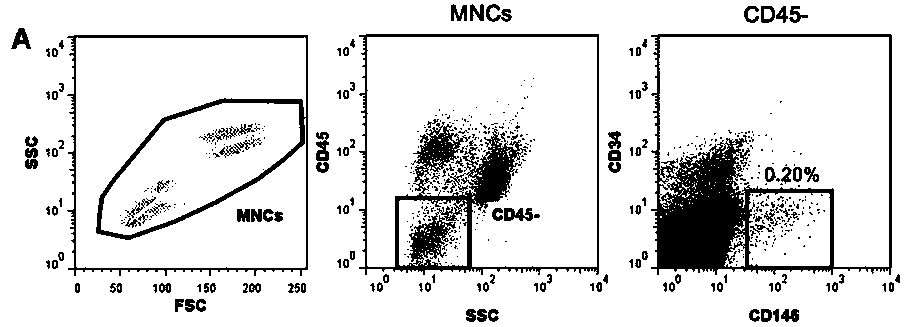
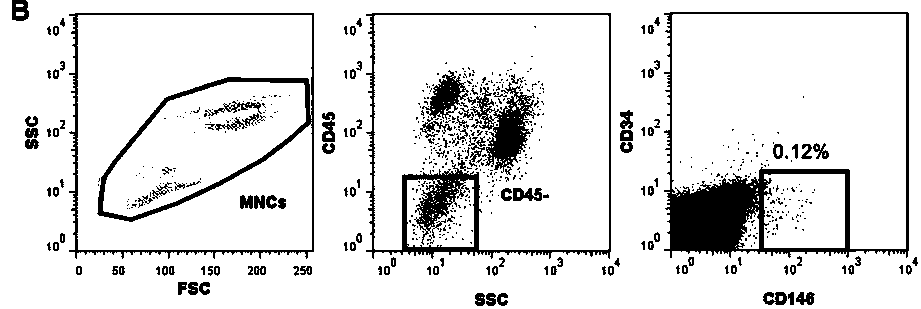
[0029] The invention provides a kit for measuring the bone marrow microenvironment of patients after hematopoietic stem cell transplantation. The application of the kit is based on flow cytometry, and the kit is configured with the following fluorescently labeled monoclonal antibody reagent: CD34-FITC , CD146-PE, CD45-PerCP and CD133-APC, see Table 1 for information on fluorescent labels and components of each monoclonal antibody.
[0030] name fluorescent label clone catalog number company CD34 FITC 8G12 348053 BD Biosciences CD146 PE 89106 550315 BD Biosciences CD45 PerCP none 347464 BD Biosciences CD133 APCs 293C3 130-090-854 Miltenyi Biotec
[0031] The kit is also configured with erythrocyte lysate, 10×PBS buffer solution with a pH of 7.2-7.4, and matching calf serum.
[0032] Specifically, the volume standard unit of each fluorescently labeled monoclonal antibody reagent in the kit is: 5 μL of CD34-FITC, ...
Embodiment 2
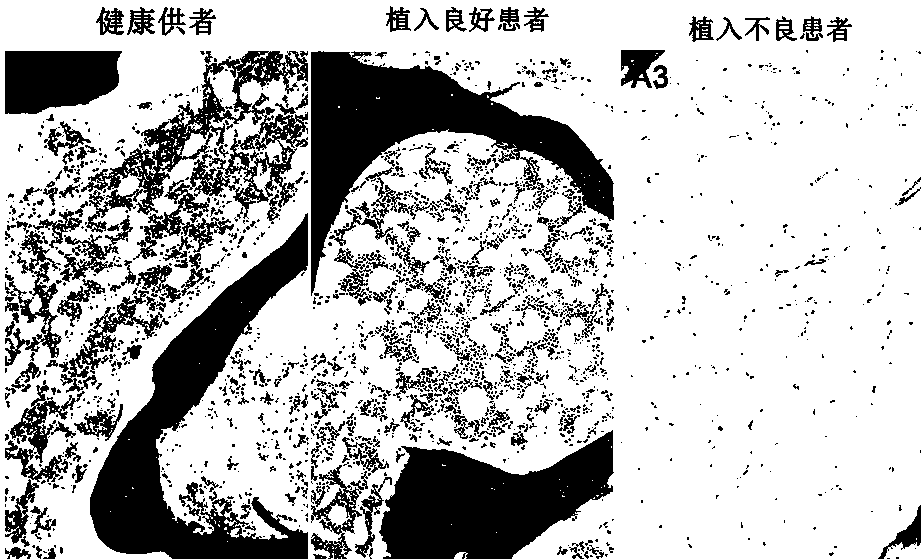
[0058] Example 2, HE staining of bone marrow microenvironment components in patients with hematological diseases after hematopoietic stem cell transplantation
[0059] 1. Fixation: take bone marrow tissue and cut into small pieces, fix in 10% neutral formalin solution for 24 hours; wash 5 times with 1×PBS buffer solution, 5 minutes each time;
[0060] 2. Dehydration: Put the fixed tissue samples in 70% ethanol for 1 hour, 80% ethanol for 1 hour, 95% ethanol I for 1 hour, 95% ethanol II for 2 hours, 95% ethanol III for 2 hours, 100% ethanol I for 1 hour hours and 100% ethanol II for 2 hours;
[0061] 3. Transparent: put the dehydrated specimen in xylene I for 10 minutes and xylene II for 20 minutes;
[0062] 4. Wax immersion: soak the transparent tissue in molten paraffin, repeat 3 times, 0.5-1 hour each time;
[0063] 5. Embedding: Pour melted new paraffin into the embedding device, put the tissue block quickly before it solidifies, and distinguish the various sides of the t...
Embodiment 3
[0071] Example 3 Immunohistochemical staining of bone marrow microenvironment components in patients with hematological diseases after hematopoietic stem cell transplantation
[0072] 1. Immunohistochemical detection of blood vessels in bone marrow tissue sections was performed with anti-human CD34 monoclonal antibody, and paraffin sections were first dewaxed and watered as in HE staining;
[0073] 2. Antigen retrieval: put slices into preheated 0.01M citrate buffer (pH 6.0) and boil for 20 minutes, then cool naturally for 20 minutes;
[0074] 3. Incubate with 3% hydrogen peroxide at room temperature for 20 minutes to eliminate the activity of endogenous peroxidase;
[0075] 4. Rinse the sections with distilled water and soak in PBS buffer for 5 minutes;
[0076] 5. Remove the PBS buffer, add 1 drop or 50ul of normal non-immune animal serum to each slice, block, incubate at room temperature for 10 minutes, pour off the serum, do not wash, add 1:100 diluted anti-human CD34 mon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com