A kind of preparation method of baquiprime
A technology of methylquinoline and intermediates, which is applied in the field of preparation of baquiprime, and achieves the effects of long reaction time, high yield and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
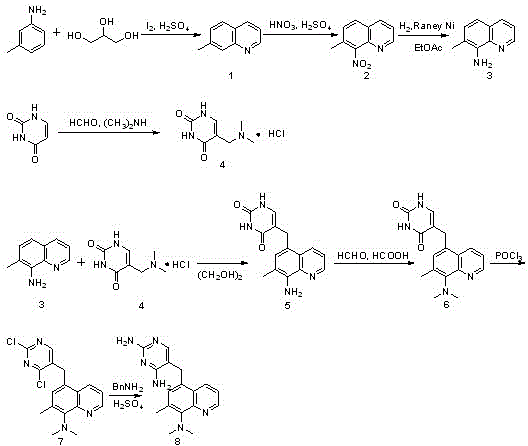
[0028] The synthetic method step of baquiprime among the present invention is as follows:
[0029] Using m-toluidine as the starting material, 8-amino-7-methylquinoline (intermediate III) was obtained through Skraup reaction, nitration and nitro reduction, and 5-dimethoxyquinoline (intermediate III) was obtained through Mannich reaction using uracil as the starting material. Methylaminomethyluracil hydrochloride (intermediate IV), and then react with 8-amino-7-methylquinoline (intermediate III) through condensation, Eschweiler-Clarke methylation, chlorination, and ammonolysis in eight steps Synthesis of baquiprine. Its specific reaction route is as follows figure 1 shown.
Embodiment 1
[0032] (1) Add glycerin (230mL, 3.1mol), m-toluidine (80.3mL, 0.75mol), iodine (9.5g, 37.5mmol) into a 1L three-necked flask, stir mechanically, and slowly add concentrated sulfuric acid (165mL, 3.1 mol), warm up to 140°C and reflux for 2 hours after dropping, add ice water 200mL to dilute after cooling, add 30% sodium hydroxide solution dropwise under ice bath, neutralize to pH>9, add ethyl acetate 500mL for extraction, water 300mL ×3 washed, added anhydrous sodium sulfate to dry, suction filtered, and evaporated to dryness of ethyl acetate to obtain brown oily liquid 1 (97g, yield 90.3%), that is, 7-methylquinoline (intermediate Ⅰ).
[0033] (2) Add concentrated sulfuric acid (230mL, 4.3mol) into a 1L three-necked flask, add intermediate I (97g, 0.68mol) under ice bath and mechanical stirring, and slowly add 65% concentrated nitric acid (54mL, 0.82mol) dropwise after dissolution ), stirred at room temperature for 1 h after dropping, poured the reaction solution into 2 L of i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com