Bidentate peptide chiral silane and chromatographic chiral stationary phase
A chiral chromatography and chirality technology, applied in the field of chromatographic separation materials, can solve the problems of short service life and weak acting force of the coated stationary phase, and achieve the effects of simple method, short synthesis route and good effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
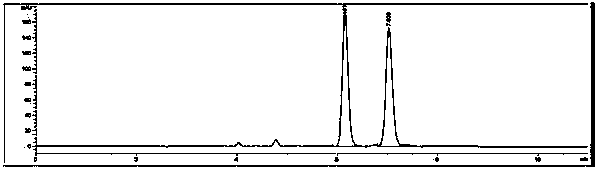
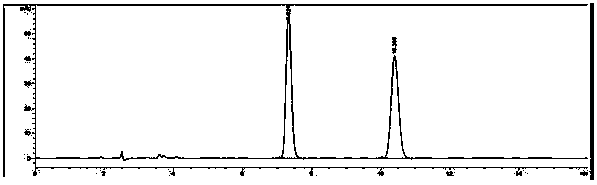
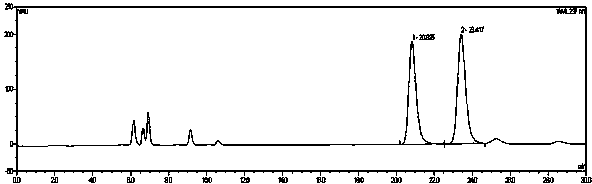
Image
Examples
Embodiment 1
[0034] Embodiment 1: Take 1,4-diphenol as raw material
[0035]
[0036] (1) Silica gel pretreatment
[0037] Kromasil Si-Tech spherical silica gel (pore size 100?, particle size 5.0 μm, specific surface area 340 m 2 / g, the metal impurity content is less than 30 ppm), according to the volume mass ratio of hydrochloric acid and silica gel 2 ml / g, mix hydrochloric acid with a volume percentage of 20% and silica gel, heat to 100°C and reflux for 4h. Filter with suction, wash with deionized water until neutral, wash with methanol, and dry at 160°C for 24h.
[0038] (2) Preparation of binaphthol-phenylalanine silane 5
[0039] ① Preparation of Intermediate 3: Weigh 8.5g (32mmol) of raw material (2) and put it into a three-neck bottle. Install a condenser tube and a dropping funnel, and feed dry nitrogen. Add 250ml of dry toluene and 8.0g of trifluoroacetic anhydride, and stir at room temperature until (2) is completely dissolved. Add 4.3g (15mmol) of raw material (1),...
Embodiment 2
[0053] Embodiment 2: Take 1,4-diphenol as raw material
[0054]
[0055]
[0056] (1) Preparation of binaphthol-dipolyphenylalanine silane 9
[0057] Preparation of Intermediate 7
[0058] Take 8.7g (15mmol) intermediate (4) and dissolve in 500ml dichloromethane, N 2 Stir under protection, then add 32mmol BOP, 32mmol HOBT, 10ml DIEA, continue stirring for 20 minutes, then add 8.5g (32mmol) raw material (2), and stir at room temperature for 2h. The reaction was detected by TLC spot plate until the raw material of the reactant disappeared. The reaction mixture was rotary evaporated to remove dichloromethane, the concentrate was added to chilled diethyl ether for precipitation, and washed three times with cold diethyl ether. The reactant intermediate (7) was purified by column chromatography, 12.9 g, with a yield of 80%.
[0059] MS: m / z 1076.25 [M+H] + , 962.04 [M-tBu+H] + . Elemental analysis: C% 73.92; H% 6.35; N% 5.42. 1 H NMR (CDCl 3 ): δ 1.26 (s, 18H); δ ...
Embodiment 3
[0070] Embodiment 3: Take 1,2-diol as raw material
[0071]
[0072] (1) Preparation of silane 15
[0073] Preparation of Intermediate 13
[0074] In the three-necked flask equipped with a condenser tube and a dropping funnel, dry nitrogen gas was introduced. Add 10.7g (32mmol) of raw material (2), 250ml of dry toluene, and 8.0g of trifluoroacetic anhydride, and stir at room temperature until (2) is completely dissolved. Add 3.2g (15mmol) of raw material (11) and stir at 40°C for 24h. Add 90ml of ethyl acetate to dilute, separate the layers, wash the ethyl acetate layer with 2N sodium hydroxide solution, hydrochloric acid and water twice, combine the organic phases, and dry over anhydrous sodium sulfate overnight. Filtration and removal of the solvent under reduced pressure gave a crude product, which was purified by flash column chromatography to obtain 11.0 g of solid intermediate (13) with a yield of 85%.
[0075] MS: m / z 708.33[M] + , 709.33 [M+H] + . Elemental...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com