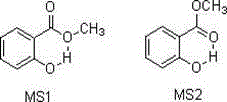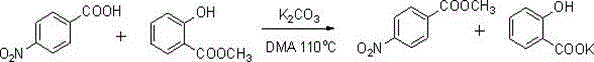Carboxylic ester preparation method
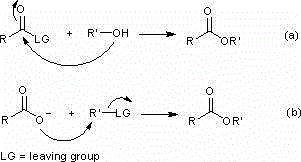
A technology for synthesizing carboxylic acid esters and carboxylic acids, which is applied in the preparation of carboxylic acid esters, the preparation of carboxylic acid amides, the formation/introduction of carboxylic acid ester groups, etc. Ester, environmental pollution, poor selectivity and other problems, to achieve the effect of a wide range of substrates, simple reaction conditions, and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 : Preparation of methyl p-nitrobenzoate
[0024]
[0025] Add 25 mmol of p-nitrobenzoic acid, 15 mmol of potassium carbonate and 50 ml of N,N-dimethylacetamide into the reaction flask, and o C for 0.5h. Then 37.5 mmol of methyl salicylate was added to continue the reaction for 20 h. After the reaction was completed, the solvent was evaporated under reduced pressure, an appropriate amount of water was added to the residue, and 12.5 mmol of potassium carbonate was added to hydrolyze the remaining methyl salicylate, which was detected by thin-layer chromatography. After the reaction is complete, extract with ethyl acetate, wash the organic phase with water and saturated sodium chloride, evaporate the solvent under reduced pressure to obtain the product carboxylate; The filter cake was solid; the filtrate was extracted with ethyl acetate, the organic phase was washed with water and saturated brine, and the solvent was evaporated under reduced pressure to ob...
Embodiment 2
[0026] Example 2 : Preparation of methyl o-methoxybenzoate
[0027]
[0028] Add 25 mmol of o-methoxybenzoic acid, 15 mmol of potassium carbonate and 50 ml of N,N-dimethylacetamide into the reaction flask, and o C for 0.5h. Then 37.5 mmol of methyl salicylate was added to continue the reaction for 24 h. After the reaction was completed, it was processed according to Example 1. Methyl p-nitrobenzoate yield 92%, 1 H NMR (600 MHz, CDCl 3 ) δ 7.80 (dd, J = 1.8, 7.8 Hz, 1H), 7.46-7.49 (m, 1H), 6.98-7.00 (m, 2H), 3.92 (s, 3H), 3.90 (s, 3H).
Embodiment 3
[0029] Example 3 : Preparation of methyl m-acetaminobenzoate
[0030]
[0031] Add 25 mmol of m-acetamidobenzoic acid, 15 mmol of potassium carbonate and 50 ml of N,N-dimethylacetamide into the reaction flask, at 110 o C for 0.5h. Then 37.5 mmol of methyl salicylate was added to continue the reaction for 20 h. After the reaction was completed, it was processed according to Example 1. The yield of methyl m-acetaminobenzoate is 86%. 1 H NMR (400 MHz, CDCl 3 ) δ 8.02 (s, 1H), 7.91 (d, J = 8.4 Hz, 1H), 7.78 (d, J = 7.6 Hz, 1H), 7.68 (br, 1H), 7.41 (t, J = 8.0 Hz, 1H), 3.90 (s, 3H), 2.21 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com