Organic compound for measuring content of metal ions in water environment and application of organic compound
A technology of organic compounds and metal ions, applied in organic chemistry, measurement devices, and analysis by making materials undergo chemical reactions, etc., can solve the problems of special ion identification, cannot be used continuously, cannot realize automatic detection, etc., and achieve mild reaction conditions. , the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
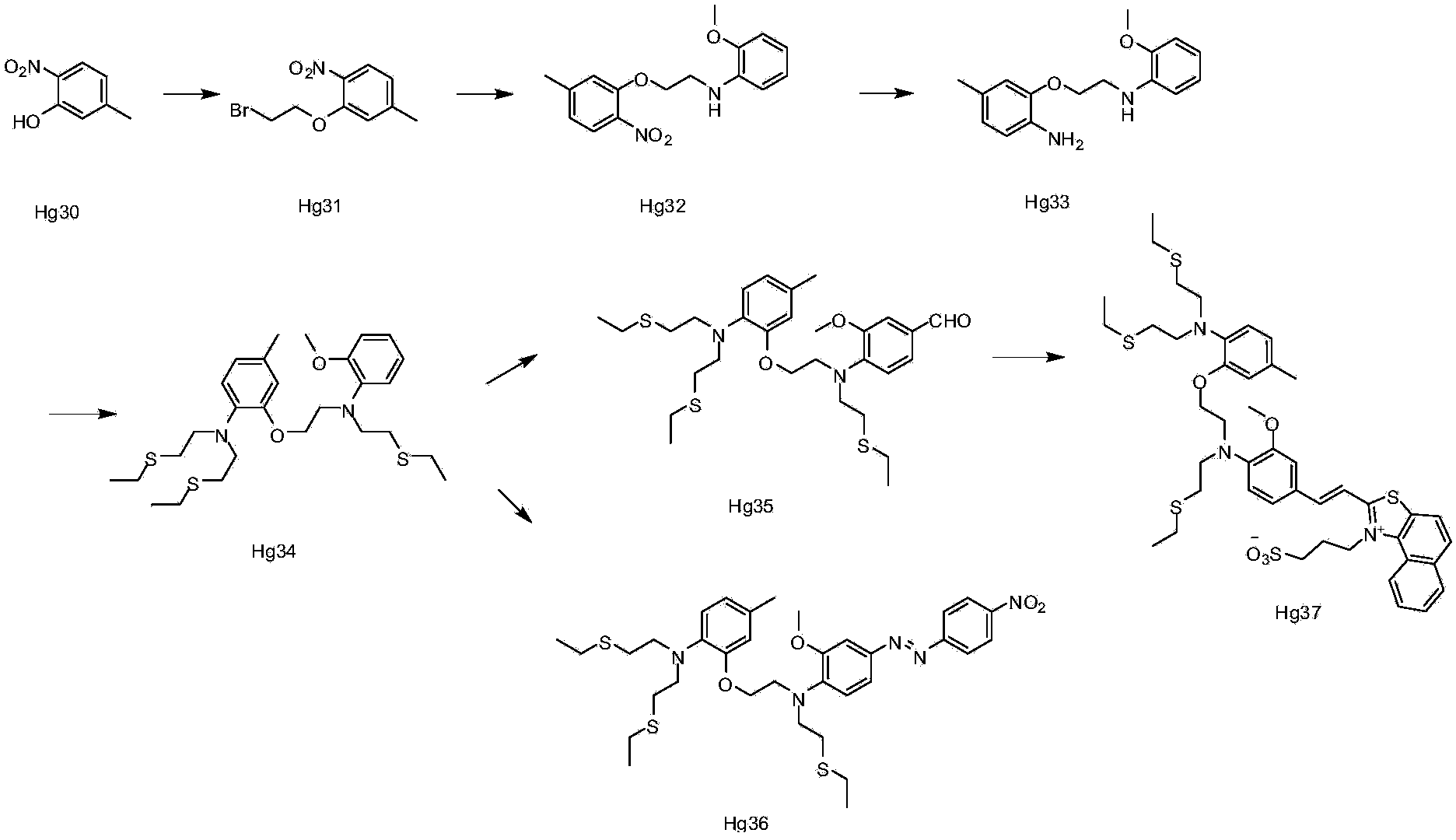
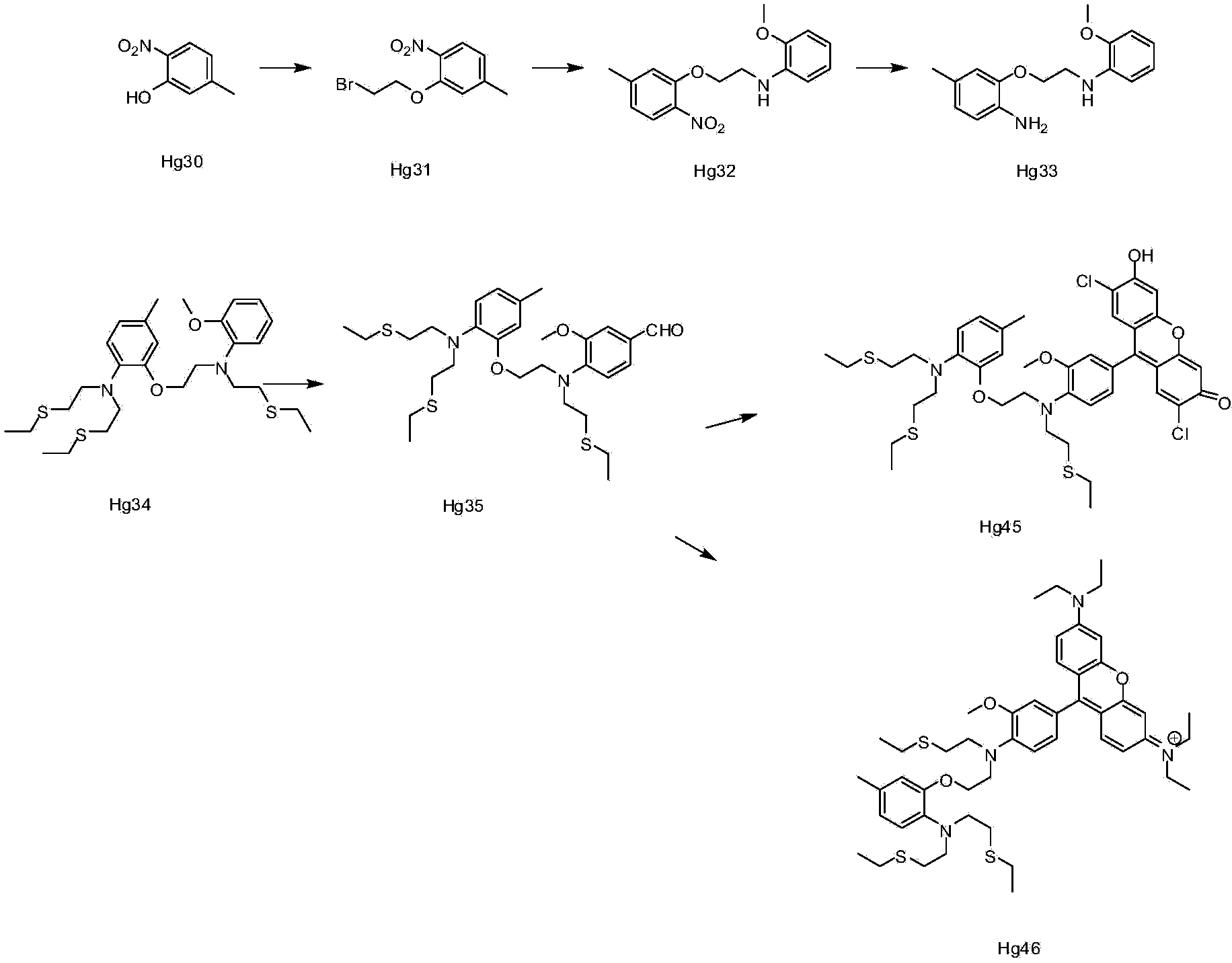
[0060] Embodiment 1: the synthesis of ion complexing group
[0061] Synthesis of compound Hg31:
[0062] Hg30 (38g0.25), 1,2-dibromoethane (108ml, 1.25mol), and potassium carbonate (34.60g, 0.25mol) were added to DMF (200ml), heated to 100°C, and kept for 2 After 1 hour, TLC detected that the reaction of the raw materials was complete, spin-dried, and recrystallized from methanol to obtain 46 g of the product. 1H NMR (CDCl3) δ = 2.40 (s, 3H), 3.65 (t, 2H) 6.85 (d, 2H), 7.75 (d, 1H).
[0063] Synthesis of compound Hg32:
[0064] At room temperature, Hg31 (80.0g, 1.5mol), o-methoxyaniline (25.0g, 1mol), potassium carbonate (56.0g, 2.0mol), potassium iodide 1(, 33.5g, 1mol) were added to 300ml of acetonitrile , refluxed overnight, TLC detection, the reaction of the raw materials was completed, spin-dried, and passed through the column to obtain 44g of the product. 1H NMR (CDCl3)8 = 2.38 (s, 3), 3.61 (t, 2H), 3.85 (s, 3H), 4.29 (t, 2H), 6.69-6.85 (m, 6H) 7.81 (d, 1H).
[0065...
Embodiment 2
[0072] Synthesis of compound Hg35:
[0073] Under ice bath, phosphorus oxychloride (14.2g, 0.09mol) was slowly added dropwise to DMF (14.2ml), stirred in ice bath for 0.5 hour, then a solution of Hg34 (10.0g, 0.018mol) in DMF (10ml) was added dropwise Add it to the above solution, turn it to room temperature and react overnight, TLC detects 50% reaction, pour it into water, extract with EA, dry, spin dry, and pass through the column to obtain 0.8g of the product. 1H NMR (CDCl3) δ=(1.20, 9H), 2.48(s, 3H) 2.57(m, 12H), 3.25(t, 4H), 3.51(t, 2H), 3.81(t, 2H), 3.88(s , 3H) 4.09 (t, 2H), 6.61-7.35 (m, 7H), 9.78 (s, 1H).
[0074] Synthesis of Compound Hg36:
[0075] Compound Hg35 (0.1g, 1.77*10 -4 mol) and 2-methyl-1-(3-sulfonylpropyl)naphtho[1,2-d]thiazolium hydroxide inner salt monohydrate (0.06g, 1.8*10 -4 mol) was added to the mixed solution of 0.5ml of acetic acid and 0.5ml of acetic anhydride, reacted at 100°C overnight, the solution turned red, TLC detected the completion ...
Embodiment 3
[0078] Synthesis of Compound Hg37:
[0079] Dissolve 2ml of p-nitroaniline (0.038g, 0.27mmol) in THF: water = 1:1 solution, add sodium nitrite (0.019g, 0.27mmol, under ice-cooling, add dropwise concentrated hydrochloric acid 0.05ml, ice-bath Stir for 1 hour, then add compound 34 (0.1g, 0.18mmol) in THF: water=1: 1 solution in 2ml, stir in ice bath for 2 hours, turn to room temperature overnight, TLC detects that new spots are generated, raw materials are not Complete the reaction, add water, extract three times with EA, dry over anhydrous sodium sulfate, and scrape to obtain an orange-yellow product. 1H NMR (CDCl3) δ=1.38 (m, 9H) 2.26 (s, 3H), 2.46-2.71 (m , 12H), 3.28(t, 4H), 3.57(t, 2H), 3.64(t, 2H), 3.96(s, 3H), 4.04(t, 2H), 6.64(m, 2H), 6.87(m, 2H), 7.05 (m, 1H), 7.50 (d, 1H), 7.98 (d, 2H), 8.37 (d, 2H).
[0080] Test of compound Hg37: 4 mg of the above product (Hg37) was dissolved in 1 mL of a mixture of methanol and water (1:1), and then the solution was prepared into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com