Method for recovering titanium from waste hydrochloric acid generated in production of titanium dioxide by chlorination process
A technology of titanium dioxide and waste hydrochloric acid, which is applied in the field of titanium recovery, can solve the problems of many stages, high price and consumption of neutral phosphorus-type extractants, achieve large extraction saturation capacity, realize resource reuse, and eliminate environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
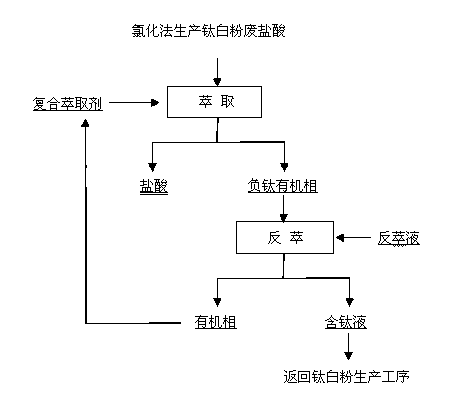
[0016] 1) Take 5L of molten salt for chlorination to produce titanium dioxide waste hydrochloric acid (component HCl: 25.2%, Ti: 21.8 g / L), and use a composite extractant (15%LK-N21 +5 % isoamyl alcohol + 80% sulfonated kerosene), the said LK-N21 has a direct or branched chain with 14-24 carbon molecules and a relative molecular mass of 300-400; the three-stage Extraction, compared to O:A=2:3, mixing time 1min, clarification time 5min, extract titanium in hydrochloric acid into organic phase, after extraction, get purified hydrochloric acid (containing Ti: 0.1 g / L) and negative titanium The organic phase;
[0017] 2) Using a mixed solution of 0.5mol / L sulfuric acid and 0.8mol / L hydrogen peroxide as the stripping agent, the negative titanium organic phase was subjected to five-stage stripping in a countercurrent contact at 10°C. Compared with O:A= 3:2, mixing time 3min, clarification time 10min, back-extract titanium from negative titanium organic phase to water phase, after b...
Embodiment 2
[0019] 1) Take 5L of boiling chlorination to produce titanium dioxide by-product hydrochloric acid (component HCl: 28.5%, Ti: 24.3 g / L), and use a composite extractant (15%LK-N21+5 %LK-N21X+80% propane), said LK-N21 is a direct chain or branched chain with 14-24 carbon molecules and a relative molecular mass of 300-400; three-stage extraction is carried out by means of countercurrent contact at 10°C, Compared with O:A=1:2, the mixing time is 3 minutes, the clarification time is 3 minutes, and purified hydrochloric acid (containing Ti: 0.05 g / L) is obtained after extraction;
[0020] 2) Using a mixed solution of 0.6mol / L sulfuric acid and 0.6mol / L hydrogen peroxide as the stripping agent, the negative titanium organic phase was subjected to five-stage stripping in a countercurrent contact at 20°C, compared to O:A=3: 2. The mixing time is 5 minutes, and the clarification time is 5 minutes. The titanium is back-extracted from the negative titanium organic phase to the water phas...
Embodiment 3
[0022] 1) Take 5L of molten salt for chlorination to produce titanium dioxide by-product hydrochloric acid (HCl: 24.5%, containing Ti: 23.6 g / L), and use a composite extractant (15%LK-N21 + 5% multi-branched hexadecyl tertiary carbon primary amine (Primene JMT molecular weight 297.5) + 80% carbon tetrachloride), the LK-N21 carbon molecule number is 14-24 straight or branched chain, relative molecular mass 300~400; three-stage extraction was carried out by means of countercurrent contact at 30°C, compared to O:A=1:2, mixing time 3min, clarification time 5min, purified hydrochloric acid (containing Ti: 0.12 g / L );
[0023] 2) Using a mixed solution of 0.5 mol / L hydrochloric acid and 1 mol / L hydrogen peroxide as the stripping agent, the negative titanium organic phase is subjected to five-stage stripping in a countercurrent contact at 30°C, compared to O:A=3:2 , the mixing time is 3 minutes, and the clarification time is 10 minutes. After stripping, a titanium-containing soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com