Device and method for cracking difluorochloromethane to prepare tetrafluoroethylene and hexafluoropropylene
A technology of difluorochloromethane and tetrafluoroethylene, applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve problems such as difficult operation and high energy consumption, and achieve high flexibility and selectivity Good, the effect of reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
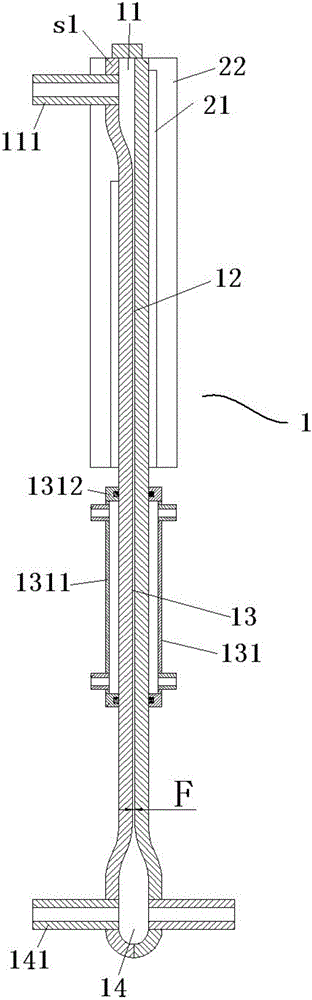
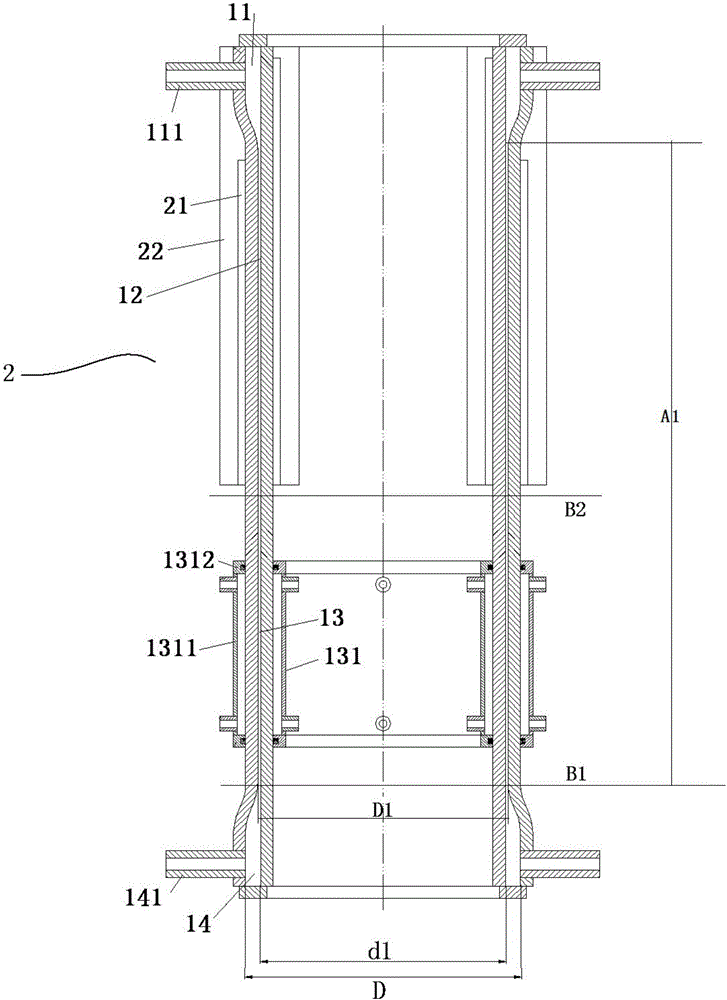
[0052] Use the above-mentioned cracking difluorochloromethane to prepare tetrafluoroethylene and hexafluoropropylene device 2 to crack R22 to prepare tetrafluoroethylene and hexafluoropropylene, including:
[0053] The cooling unit is in the figure 2 location shown.
[0054] The heating body heats the reaction chamber of the inner and outer circular tubes to 650°C; the R22 gas that has been preheated to 450°C enters the device from the gas inlet, and is heated rapidly to produce tetrafluoroethylene and hexafluoropropylene.
[0055] After the gas in the device is indirectly cooled by the water in the cooling chamber, a mixture is obtained at the product gas outlet.
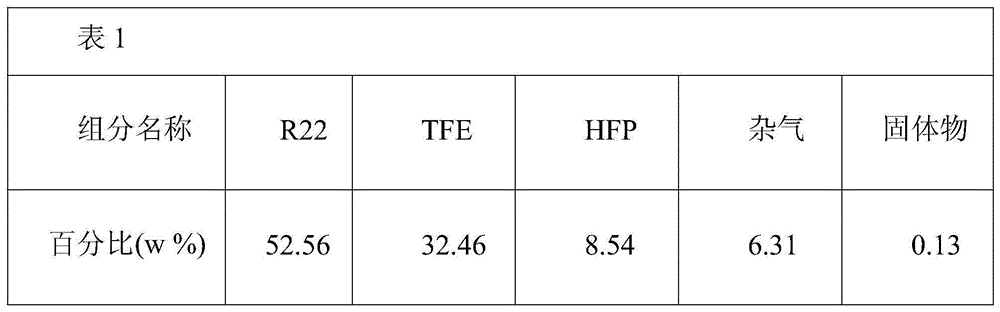
[0056] After chemical analysis, the components are shown in Table 1.
[0057]
Embodiment 2
[0059] Use the above-mentioned cracking difluorochloromethane to prepare tetrafluoroethylene and hexafluoropropylene device 2 to crack R22 to prepare tetrafluoroethylene and hexafluoropropylene, including:
[0060] The lower part of the cooling unit is in the figure 2 B1 position shown.
[0061] The heating body heats the reaction chamber of the inner and outer circular tubes to 850°C; the R22 gas that has been preheated to 450°C enters the device from the gas inlet, and is heated rapidly to produce tetrafluoroethylene and hexafluoropropylene.
[0062] After the gas in the device is indirectly cooled by the water in the cooling chamber, a mixture is obtained at the product gas outlet.
[0063] After chemical analysis, the components are shown in Table 2.
[0064]
Embodiment 3
[0066] Use the above-mentioned cracking difluorochloromethane to prepare tetrafluoroethylene and hexafluoropropylene device 2 to crack R22 to prepare tetrafluoroethylene and hexafluoropropylene, including:
[0067] The top of the cooling unit is figure 2 B2 position shown in .
[0068] The heating body heats the reaction chamber of the inner and outer circular tubes to 850°C; the R22 gas that has been preheated to 450°C enters the device from the gas inlet, and is heated rapidly to produce tetrafluoroethylene and hexafluoropropylene.
[0069] After the gas in the device is indirectly cooled by the water in the cooling chamber, a mixture is obtained at the product gas outlet.
[0070] After chemical analysis, the components are shown in Table 3.
[0071]
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com