Preparation method for phosphate buffer solution
A phosphate buffer and buffer solution technology, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, inorganic non-active ingredients, etc., can solve problems such as red blood cell atrophy, affect the efficacy of sodium hyaluronate, dehydration, etc., and shorten the service life , reduce clinical redness and pain, improve product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
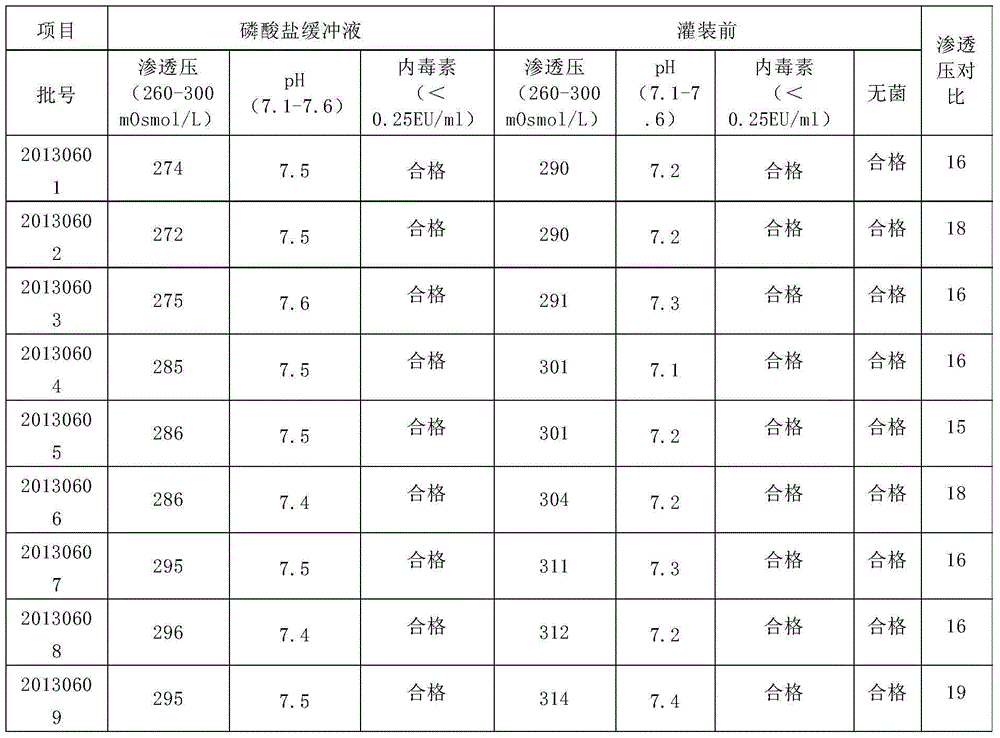
[0026] Add 60kg of water for injection into the liquid preparation tank, set the stirring frequency to 40HZ, start stirring, and the stirring speed is 100 rpm, weigh 480g of sodium chloride and add it to the PBS tank, stir while adding, and then add the , 15.0 g of disodium hydrogen phosphate and 1.8 g of sodium dihydrogen phosphate after 2 hours of dry heat treatment, stirred for 5 minutes, and 30 kg of phosphate buffer solution was sterilized and filtered through two series-connected filters of 0.2 μm in diameter Bacteria preparation tank, sampling inspection. Weigh 306g of dry and purified sterile sodium hyaluronate and slowly add it into the buffer solution. After dissolving evenly, the pre-filling solution is obtained. Take samples for testing and make three batches in succession. The batch numbers are 20130601, 20130602, and 20130603. The buffer solution of batch number 20130601 has an osmotic pressure of 274mOsmol / L, a pH of 7.5, and bacterial endotoxins less than 0.25E...
Embodiment 2
[0028] Add 60kg of water for injection into the liquid preparation tank, set the stirring frequency to 45HZ, start stirring, and the stirring speed is 125 rpm, weigh 495g of sodium chloride and add it to the PBS tank, stir while adding, and then add the 16.8 g of disodium hydrogen phosphate and 2.4 g of sodium dihydrogen phosphate after 2 hours of dry heat treatment were stirred for 8 minutes, and 30 kg of phosphate buffer solution was sterilized and filtered through two series-connected filters of 0.2 μm in diameter. Bacteria preparation tank, sampling inspection. Weigh 306g of dry and purified sterile sodium hyaluronate and slowly add it to the buffer solution. After dissolving evenly, the pre-filling solution is obtained. Samples are taken for testing and three batches are made in succession. The batch numbers are 20130604, 20130605, and 20130606. The buffer solution of batch number 20130604 has an osmotic pressure of 285mOsmol / L, a pH of 7.5, and bacterial endotoxins less ...
Embodiment 3
[0030] Add 60kg of water for injection into the liquid preparation tank, set the stirring frequency to 50HZ, start stirring, and the stirring speed is 150 rpm, weigh 510g of sodium chloride and add it to the PBS tank, stir while adding, and then add dihydrogen phosphate Sodium 18.0g and sodium dihydrogen phosphate 3.0g were stirred for 10 minutes, and 30kg of phosphate buffer solution was sterilized and filtered through two series-connected filters with a pore size of 0.2um, and poured into a sterile liquid preparation tank for sampling and inspection. Weigh 306g of dry and purified sterile sodium hyaluronate and slowly add it into the buffer solution. After dissolving evenly, the pre-filling solution is obtained. Samples are taken for testing, and three batches are made in succession. The batch numbers are 20130607, 20130608, and 20130609. The buffer solution of batch number 20130607 has an osmotic pressure of 295mOsmol / L, a pH of 7.5, and bacterial endotoxins less than 0.25EU...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com