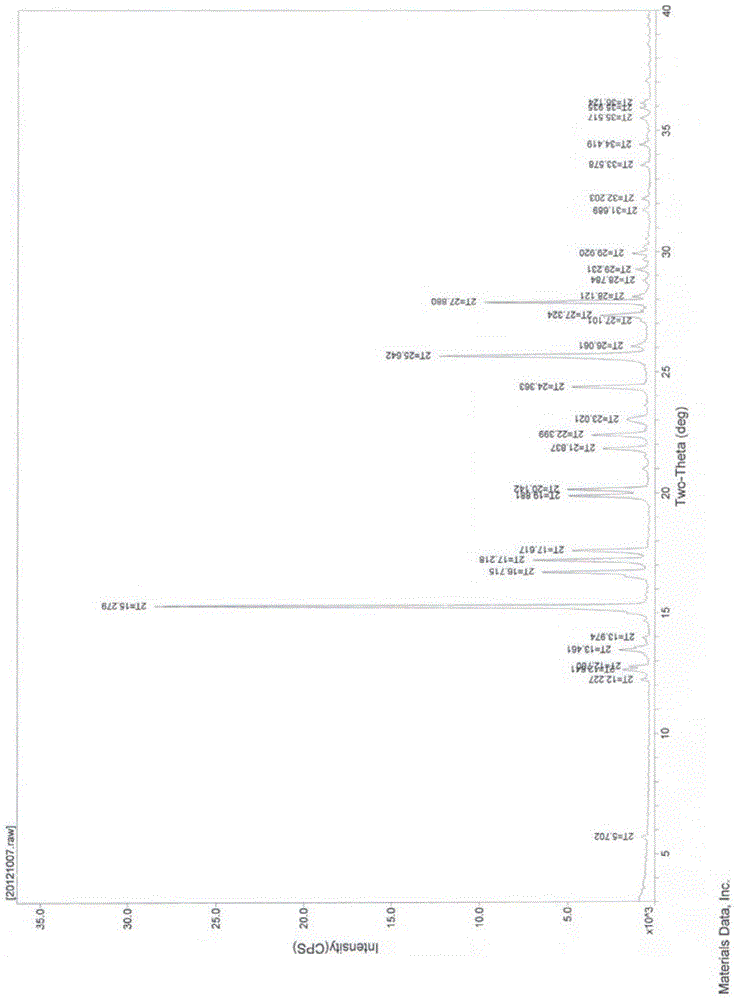
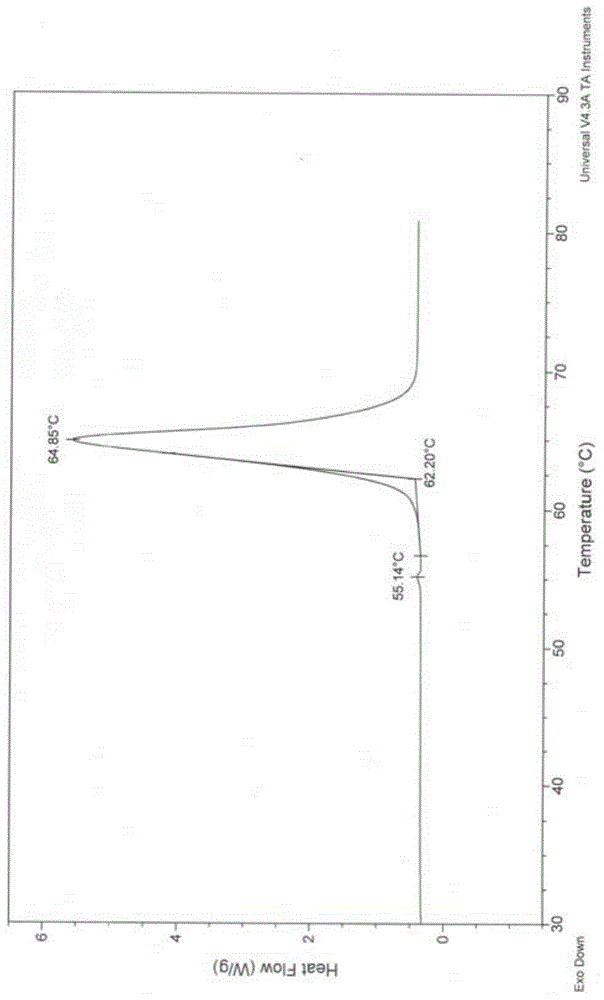
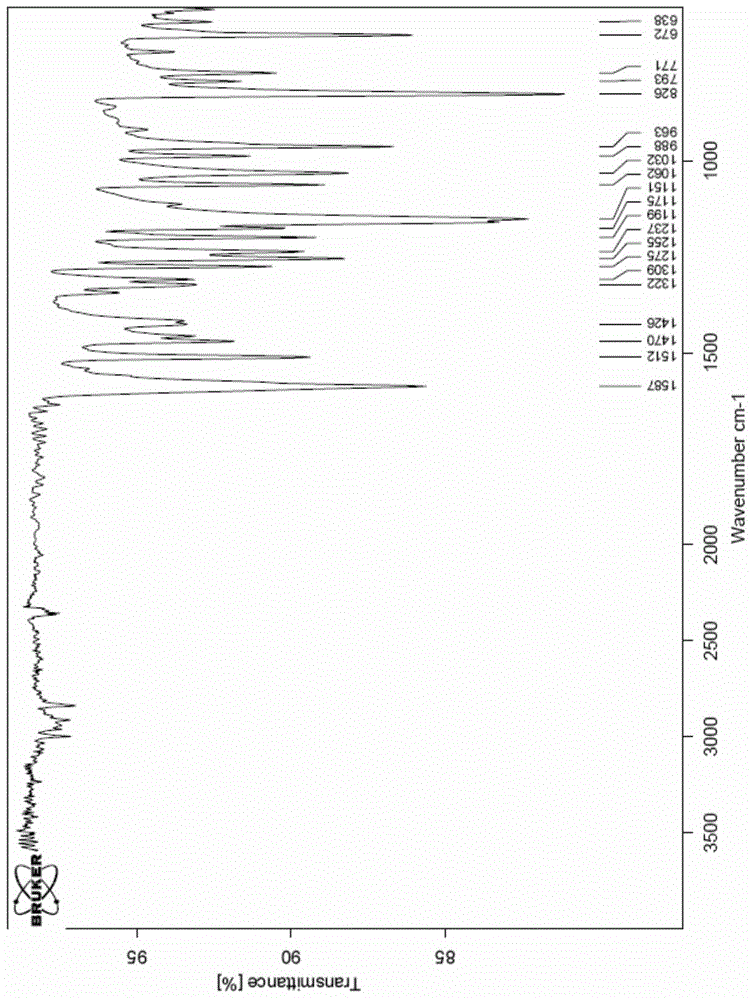
A new crystal form of resveratrol trimethyl ether and its preparation method
A technology for resveratrol trimethyl ether and crystal form, which is applied to the new crystal form of resveratrol trimethyl ether and the field of preparation thereof, can solve the problems of difficult drying, troublesome post-processing, poor stability and the like, and achieves easy drying , the effect of good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation of resveratrol trimethyl ether
[0036] Add 40g (0.214mol) of 3,5-dimethoxybenzyl chloride and 54g (0.428mol) of trimethyl phosphite into a 250ml four-necked flask, heat to an oil bath temperature of 140°C, follow the reaction by TLC, and react after about 24 hours Completely, use water pump (oil bath temperature at about 120°C) and Roots pump (oil bath temperature at about 120°C) to recover and remove trimethyl phosphite under reduced pressure to obtain 3,5-dimethoxybenzyl phosphoric acid The remaining reaction liquid of dimethyl ester is about 57g.
[0037] Add 27g of DMSO and 26.9g (0.198mol) of p-methoxybenzaldehyde to the remaining 57g of the reaction solution, cool it to 10°C in a water bath, and start adding KOH after the temperature stabilizes, adding about 2g each time, once every 20 minutes, and adding a total of 19g (0.339mol). Maintain the reaction temperature at 10°C. During the reaction, solids are gradually precipitated. TLC is...
Embodiment 2
[0057] Embodiment 2: Preparation of resveratrol trimethyl ether
[0058] The amount of trimethyl phosphite was changed to 40.5 g (0.321 mol), and the other reaction conditions were the same as in Example 1 to obtain 48 g of resveratrol trimethyl ether with a yield of 83.1%.
Embodiment 3
[0059] Embodiment 3: Preparation of resveratrol trimethyl ether
[0060] The amount of trimethyl phosphite was changed to 108 g (0.857 mol), and the remaining reaction conditions were the same as in Example 1 to obtain 52.2 g of resveratrol trimethyl ether with a yield of 90.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com