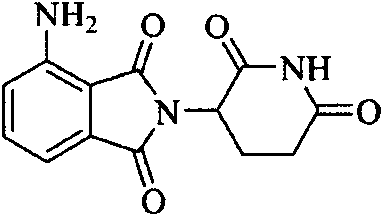
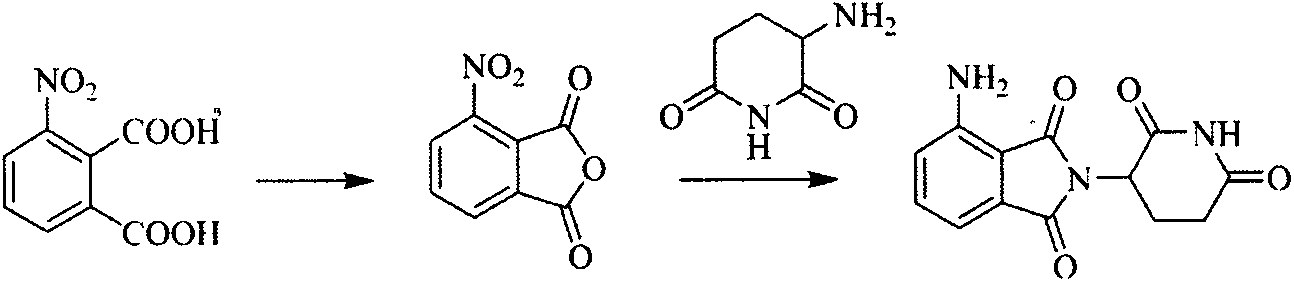
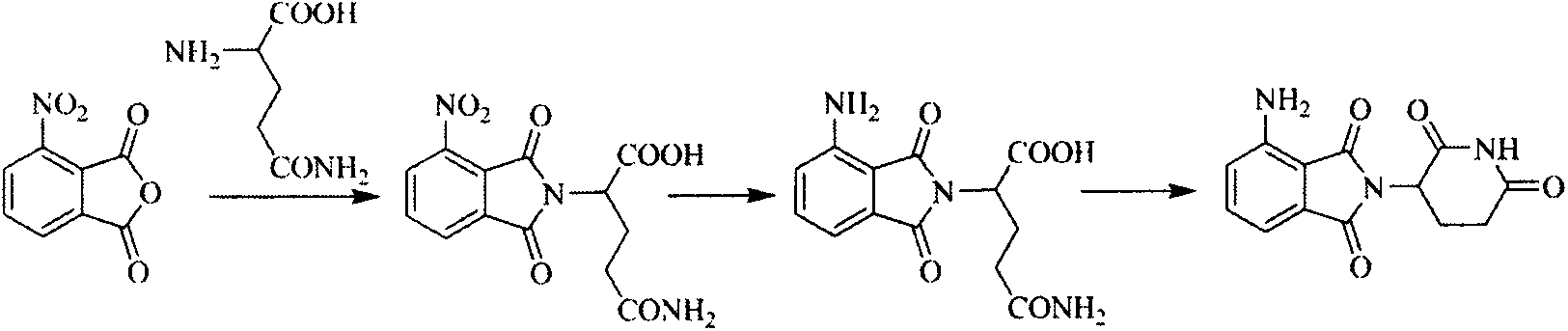
Preparation method of N-(2, 6-dioxo-3-piperidyl) phthalimide compound
A technology of nitrophthalic acid and aminoglutarimide, applied in the field of pomalidomide preparation, can solve the problems of easy moisture absorption, short preparation route, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of 3-nitro-N-(2,6-dioxo-3-piperidinyl)-phthalimide
[0049] In a 2L reaction flask, add 3-nitrophthalic acid (150g, 0.7mol), 3-amino-2,6-piperidinedione hydrochloride (90g, 0.55mol), 100g molecular sieve and 1.6L For glacial acetic acid, add anhydrous sodium acetate (50 g, 0.37 mol) after heating to 105-115 °C, keep the temperature at this temperature for 3-4 hours, and stop the reaction after TLC detects that the reaction is complete. Suction filtration and thorough drying gave off-white solid (154 g, 93%), mp 282-283°C.
[0050] 1 H NMR (DMSO-d 6 )δ: 11.19 (s, 1H, CONHCO), 8.32 (d, 1H, CHCNO 2 ), 8.24 (d, 1H, CHCCO), 8.10 (d, 1H, CHC H CH), 5.14~5.21(m, 1H, COC H CH a h b ), 2.81~2.86 (m, 1H, C H a h b CO), 2.55~2.60(m, 1H, COCHC H a h b ), 2.43~2.51 (m, 1H, CH a H b CO), 2.02~2.06(m, 1H, COCHCH a H b ).
Embodiment 2
[0051] Embodiment 2: the preparation of pomalidomide
[0052] Thoroughly dissolve 3-nitro-N-(2,6-dioxo-3-piperidinyl)-phthalimide (80 g, 0.26 mol) in 1.2 L of N, N-dimethyl Add 10 g of palladium on carbon to the formamide, transfer to a hydrogenation kettle, and react for 12 hours at a hydrogen pressure of 0.5-1.0 MPa. After the reaction was completed, the palladium-carbon was filtered off, 120 g of activated carbon was added to the filtrate, stirred for 0.5 h, and filtered with suction, an appropriate amount of pure water was added dropwise to the filtrate, stirred for 1 h, filtered with suction, and fully dried to obtain the crude product (68 g, 95%). After beating and washing with hot water, a yellow solid was obtained, mp>300°C.
[0053] 1 HNMR (DMSO-d 6 )δ.1112(s, 1H, CONHCO), 745(t, 1H, CHC H CH), 7.01(d, 1H, CHCCO), 7.01(d, 1H, C H CNH 2 ), 6.54 (s, 2H, NH 2 ), 5.03~5.08 (m, 1H, COC H CH a h b ), 2.85~2.89 (m, 1H, C H a h b CO), 2.59~2.63(m, 1H, COCHC H a...
Embodiment 3
[0055] Embodiment 3: the preparation of compound 2
[0056]
[0057] Add 0.8mol of 3-nitrophthalic acid, 0.55mol of compound 1 and 1.5L of N,N-dimethylformamide to a 2L reaction flask in sequence, add 70g of anhydrous sodium acetate after heating to 120°C, and keep the The reaction was carried out at high temperature for 5 h, and the reaction was stopped after the completion of the reaction detected by TLC. Suction filtration, fully dried to obtain off-white solid (yield 80%).
[0058] 1 H NMR (DMSO-d 6 )δ: 11.19 (s, 1H, CONHCO), 8.32 (d, 1H, CHCNO 2 ), 8.24 (d, 1H, CHCCO), 8.10 (d, 1H, CHC H CH), 7.30~7.40(m, 5H, CHCHCHCHCH ), 5.14~5.21(m, 1H, COC H CH a h b ), 4.32(d, 2H, CC H 2 C), 2.81 ~ 2.86 (m, 1H, C H a h b CO), 2.55~2.60(m, 1H, COCHC H a h b ), 2.43~2.51 (m, 1H, CH a H b CO), 2.02~2.06(m, 1H, COCHCH a H b ).
[0059] LC-MS(ESI)(m / z):394[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com