Iridium-containing hydrosilylation catalyst and composition containing same
A technology for hydrosilylation reaction and composition, applied in catalyst activation/preparation, iron organic compounds, indium organic compounds, etc., can solve the problems of difficult preparation, difficult to obtain metals, expensive and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0189] The preparation method of the resin is well known in the art. For example, the resin can be prepared by treating the resin copolymer produced by the silica hydrosol capping method described by Daudt et al. with at least one alkenyl group-containing capping agent. The method described by Daudt et al. is disclosed in US Patent 2,676,182.
[0190] The method of Daudt et al. involves reacting silica hydrosols with hydrolyzable triorganosilanes (such as trimethylchlorosilane), siloxanes (such as hexamethyldisiloxane), or mixtures thereof under acidic conditions , And the recovery of copolymers with M units and Q units. The resulting copolymer usually contains 2 to 5% by weight of hydroxyl groups.
[0191] The resin (usually containing less than 2% of silicon-bonded hydroxyl groups) can be prepared by the following method: the product described by Daudt et al. with an unsaturated organic group-containing end-capping agent and a non-aliphatic unsaturated group-free The capping a...
example
[0300] These examples are intended to illustrate some embodiments of the invention and should not be construed as limiting the scope of the invention described in the claims. The following ingredients are used in the examples.
[0301] The aliphatic unsaturated compound can be styrene (B1), 1-octene (B2) or 1-hexene (B3), all of which are also available from Sigma-Aldrich. Alternatively, the aliphatic unsaturated compound may be (B4) vinyl-terminated polydimethylsiloxane containing 2.6 meq of silicon-bonded vinyl and having a Mw of 9400 and a viscosity of 200 cSt, which is commercially available as DMS-V22 From the Gellert Company of Morrisville, Pennsylvania, USA. The SiH functional compound can be (C1) trimethylsiloxy terminated poly(methylhydrogen)siloxane ("MD H M"), which has an Mw in the range of 1,800 to 2,100 and a SiH content of 2.6 meq, is also commercially available from Gelest Company as HMS-992. Alternatively, the SiH functional compound can be (C2)phenylsilane (" ...
example 1
[0305] Example 1-Formation of metal-ligand complex
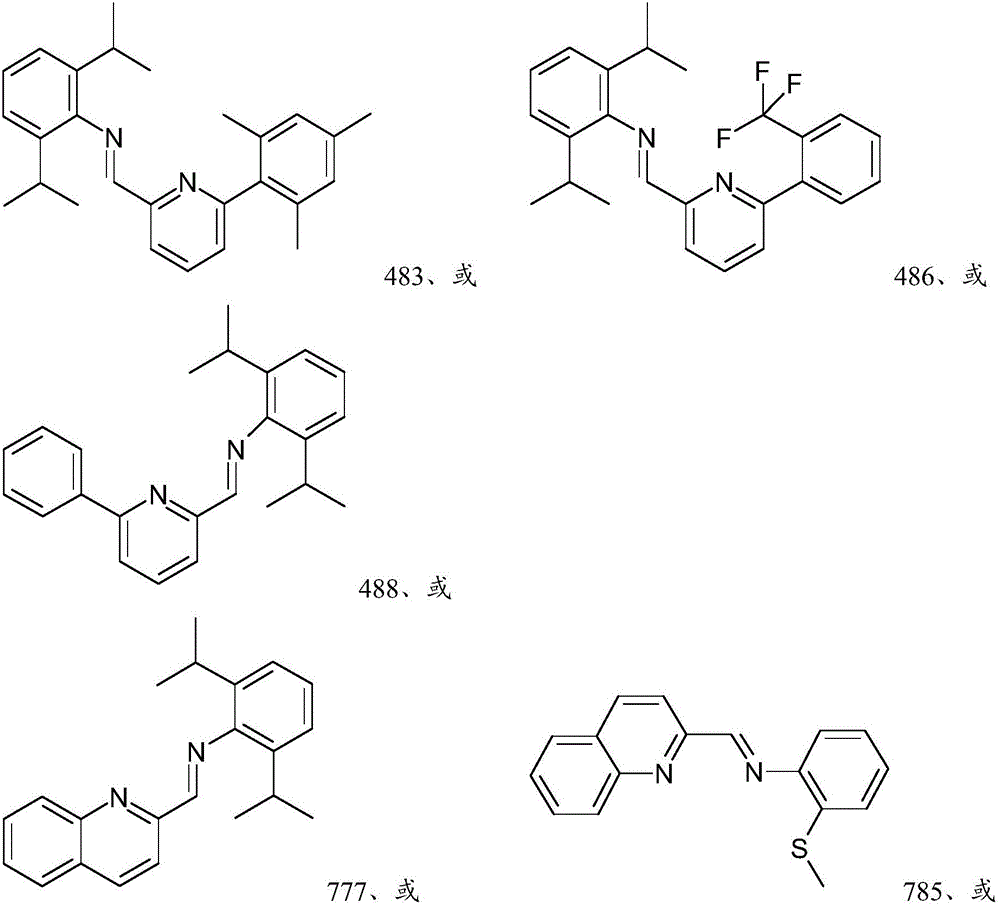
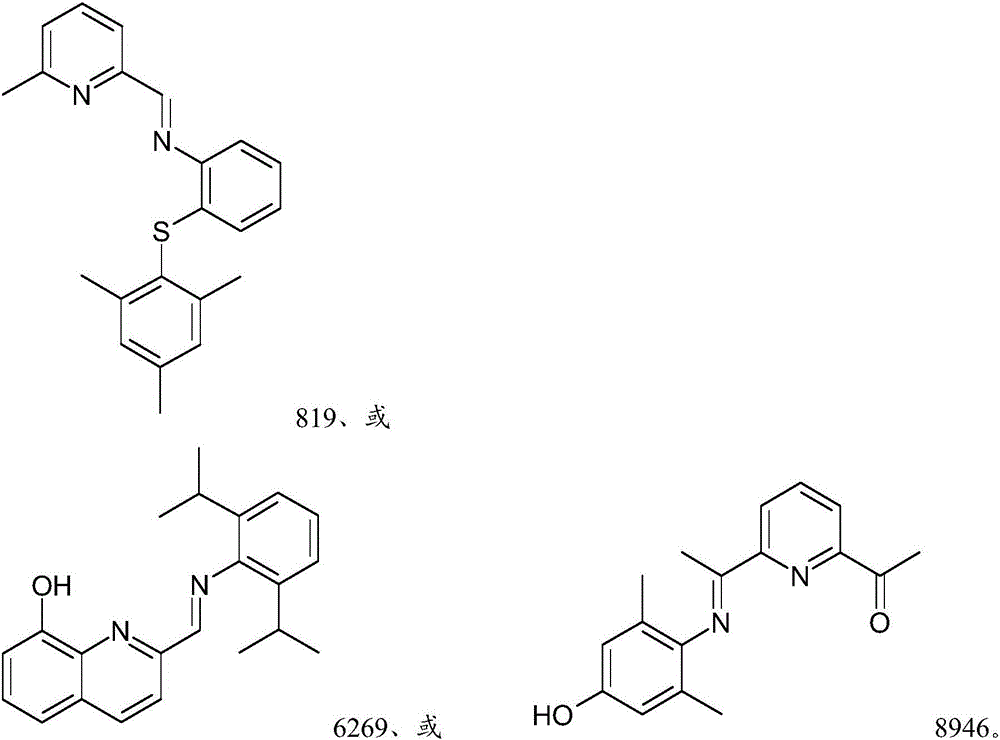
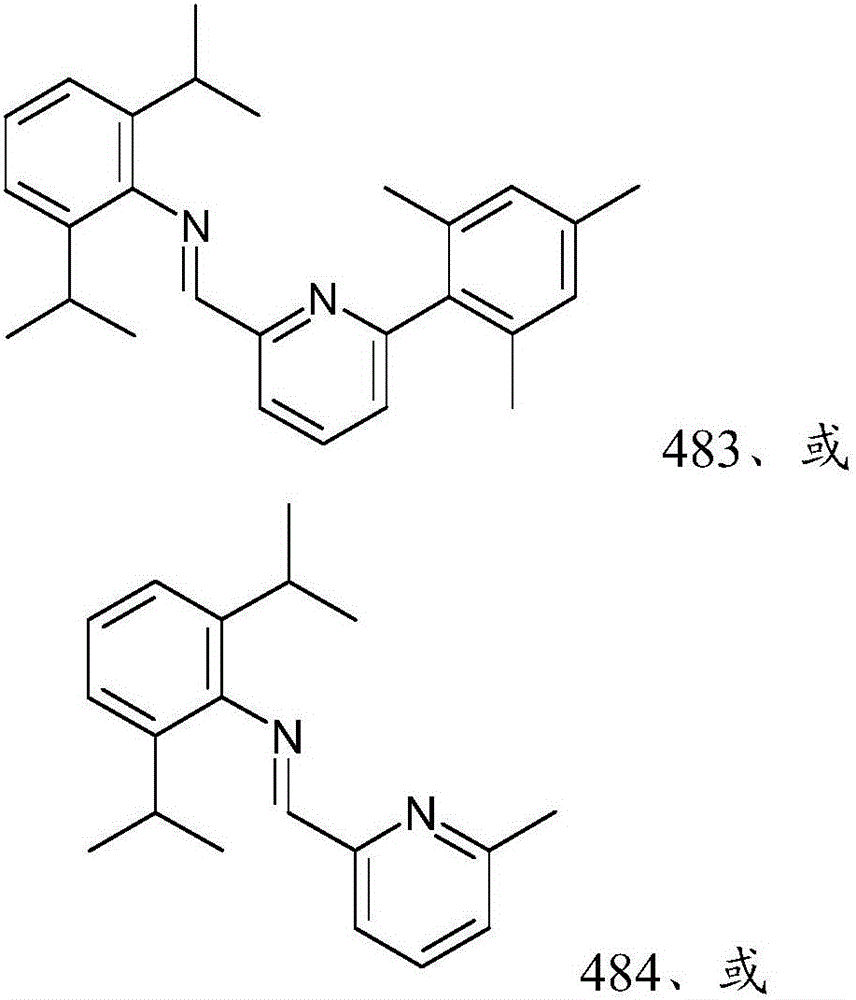
[0306] The precursor solution was prepared by the following method: 0.025 mol / L ( M ) The concentration of Ir precursor is mixed with THF, or if the precursor is not soluble in THF, the precursor is mixed with a suitable solvent selected from the group consisting of dimethyl sulfoxide (DMSO), toluene and hexane for dissolving the ligand . The Ir precursors used were iridium(III) chloride (Ir-1 precursor) and 1,5-cyclooctadiene iridium(I) chloride dimer (Ir-2 precursor). Also passed 0.025 M The concentration of the ligand was mixed with THF to prepare the solution of each ligand shown in Table 2 above. Each ligand solution prepared above was divided into 2 milliliters (mL) vials at 85 microliters (μL) per vial. In order to prepare a sample to be evaluated as component (A), one of the above-mentioned metal precursor solutions was added to the vial containing the ligand, and another 85 microliters (μL) of THF was added and kept...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com