Method for preparing midbody (2S)-N-chloracetyl-2-cyano tetrahydropyrrole of vildagliptin
A technology of cyanotetrahydropyrrole and chloroacetyl, which is applied in the field of medicine, can solve the problems of high toxicity, unsuitable for industrialization, and cumbersome post-processing, and achieve the effects of low pollution, low raw material cost, and small damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
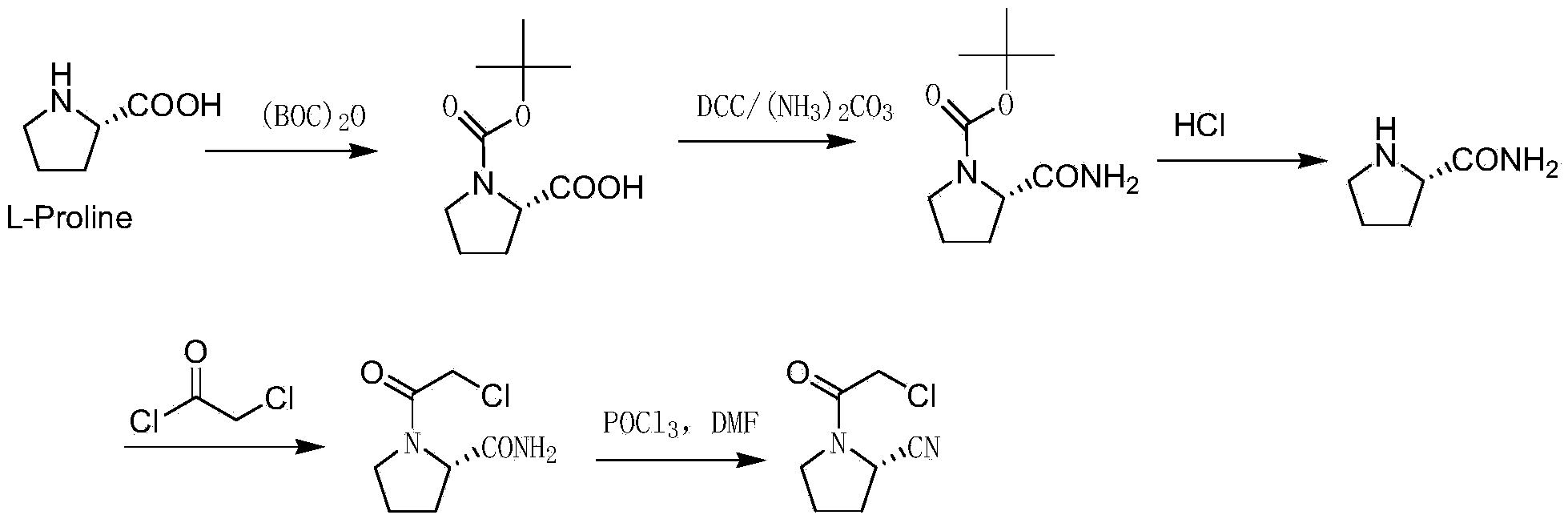
[0033] Embodiment 1: A preparation method of vildagliptin intermediate (2S)-N-chloroacetyl-2-cyanotetrahydropyrrole, specifically comprising the following steps:
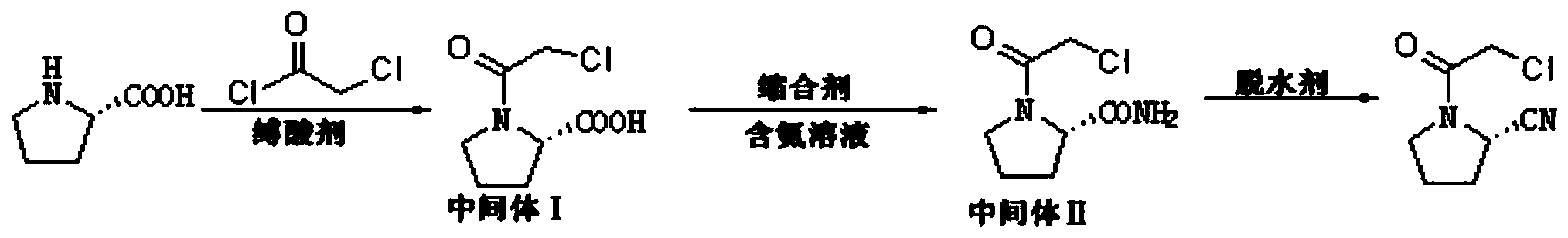
[0034] S1. Synthesis of intermediate Ⅰ (2S)-N-chloroacetyl-2-carboxylic acid tetrahydropyrrole:
[0035] Suspend 50 g of L-proline in the solvent toluene, add the acid-binding agent triethylamine, slowly add chloroacetyl chloride at room temperature, stir and react in an oil bath at 0°C for 1 hour, track the reaction by TLC, and cool the reaction solution to At room temperature, add 200ml of ethyl acetate for separation and extraction, the organic phase was first washed with saturated brine, then dried with anhydrous sodium sulfate, and finally concentrated to obtain the crude product intermediate Ⅰ (2S)-N-chloroacetyl-2-carboxylic acid Tetrahydropyrrole, the crude product intermediate I was recrystallized with ethyl acetate to obtain 65.3 g of refined intermediate I (2S)-N-chloroacetyl-2-carboxylic acid tetrahydrop...
Embodiment 2
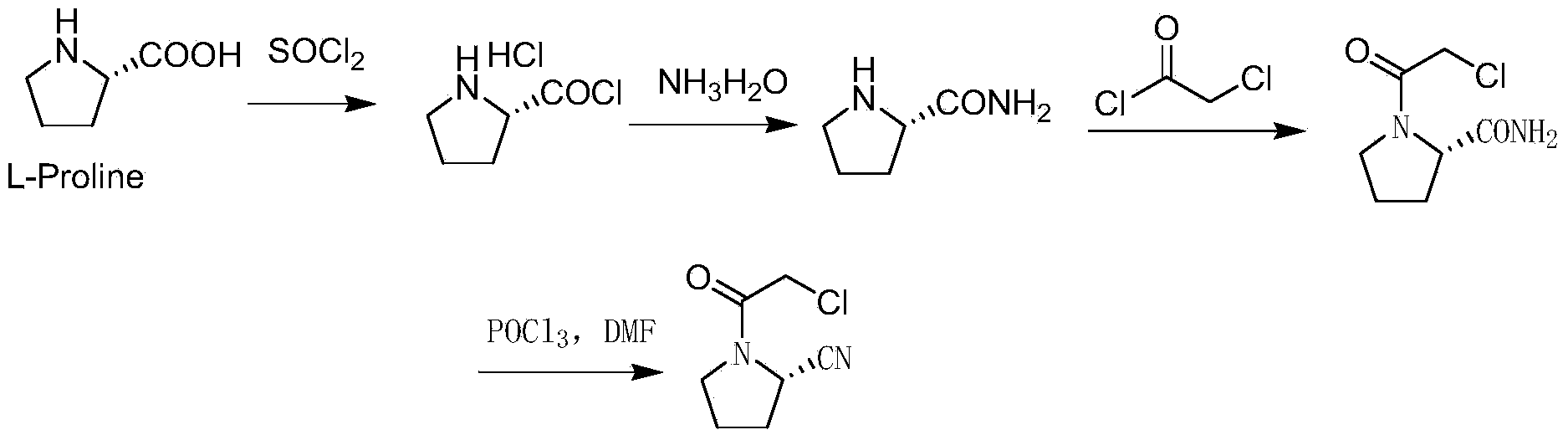
[0040] Embodiment 2: A preparation method of vildagliptin intermediate (2S)-N-chloroacetyl-2-cyanotetrahydropyrrole, specifically comprising the following steps:
[0041] S1. Synthesis of intermediate Ⅰ (2S)-N-chloroacetyl-2-carboxylic acid tetrahydropyrrole:
[0042] Suspend 50 g of L-proline in the solvent chloroform, add the acid-binding agent diisopropylamine, slowly add chloroacetyl chloride at room temperature, stir and react in an oil bath at 150°C for 72 hours, track the reaction by TLC, and cool the reaction solution to At room temperature, 200ml of ethyl acetate was added for separation and extraction, the organic phase was first washed with saturated brine, then dried with anhydrous sodium sulfate, and finally concentrated to obtain the crude product intermediate Ⅰ (2S)-N-chloroacetyl-2-carboxylic acid Tetrahydropyrrole, the crude product intermediate I was recrystallized with ethyl acetate to obtain 50 g of refined intermediate I (2S)-N-chloroacetyl-2-carboxylic ac...
Embodiment 3
[0047] Embodiment 3: A preparation method of vildagliptin intermediate (2S)-N-chloroacetyl-2-cyanotetrahydropyrrole, specifically comprising the following steps:
[0048] S1. Synthesis of intermediate Ⅰ (2S)-N-chloroacetyl-2-carboxylic acid tetrahydropyrrole:
[0049] Suspend 50g of L-proline in the solvent methanol, add the acid-binding agent diisopropylethylamine, slowly add chloroacetyl chloride at room temperature, stir and react in an oil bath at 15°C for 10h, follow the reaction by TLC, and after the reaction is completed, the reaction The solution was cooled to room temperature, and 200ml of ethyl acetate was added for separation and extraction. The organic phase was first washed with saturated brine, then dried with anhydrous sodium sulfate, and finally concentrated to obtain the crude product intermediate Ⅰ (2S)-N-chloroacetyl-2 -Carboxylic acid tetrahydropyrrole, the crude product intermediate I was recrystallized with ethyl acetate to obtain refined intermediate I (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com