T7 peptide -modified ZL006 long-circulating liposome and preparation method thereof
A long-circulating liposome and phospholipid technology, applied in the field of medicine, can solve the problems such as the limited ability of ZL006 to pass through the blood-brain barrier, the reduction of drug efficacy, etc., and achieve the advantages of reducing systemic side effects, simple and easy preparation method, and good stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of the ZL006 long-circulation liposome modified by the above-mentioned T7 peptide comprises the following steps:
[0033] Step 1, dissolve maleimide-polyethylene glycol-phosphatidylethanolamine in N,N-dimethylformamide to obtain maleimide-polyethylene glycol-phosphatidylethanolamine solution, and dissolve T7 peptide The T7 peptide solution was obtained in a phosphate buffer solution with pH=6.5-7.5; the maleimide-polyethylene glycol-phosphatidylethanolamine solution and the T7 peptide solution were respectively added dropwise to the phosphate buffer solution with a pH=6.5-7.5 In the liquid, protected by nitrogen gas, stirred for 10-14 hours to obtain a reaction liquid, wherein the molar ratio of T7 peptide to maleimide-polyethylene glycol-phosphatidylethanolamine is 1:1-5:1;
[0034] Step 2. Evaporate the reaction solution obtained in step 1 to remove N,N-dimethylformamide, then dialyze to remove unreacted T7 peptide and maleimide-polyethylene gly...
Embodiment 1
[0037] Synthesis, Purification and Characterization of T7 Peptide-Polyethylene Glycol 2000-Distearoyl Phosphatidylethanolamine (HAIYPRH-PEG2000-DSPE)
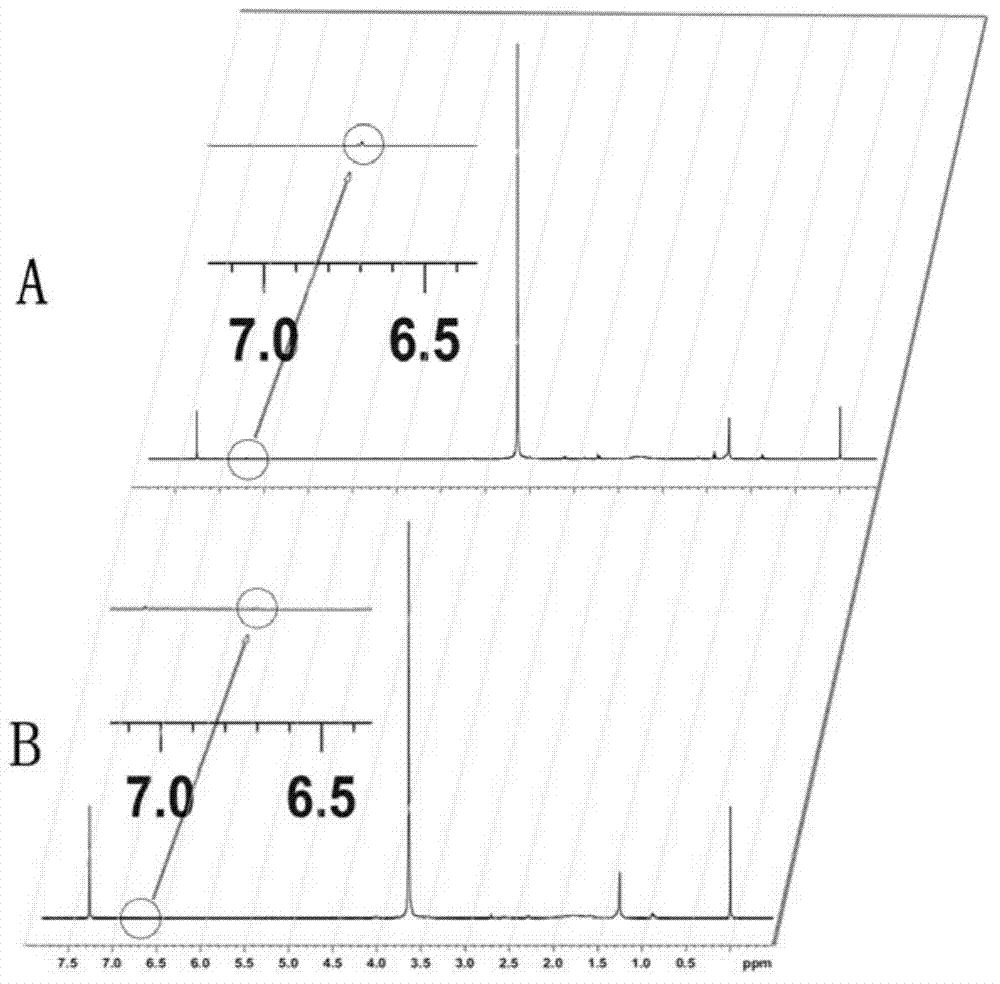
[0038] Take 8mg of maleimide-polyethylene glycol 2000-distearoylphosphatidylethanolamine (Mal-PEG2000-DSPE), dissolve it in 1ml N,N-dimethylformamide (DMF); take another 10mg of T7 peptide, Dissolve in 1ml pH7.0 phosphate buffer. The two solutions were added dropwise to 10 ml of pH 7.0 phosphate buffer, under nitrogen protection, and magnetically stirred overnight to obtain the primary product. The initial product was removed by a 30°C rotary evaporator to remove DMF while concentrating the volume to obtain the product. The product was then dialyzed (molecular weight cut-off 3.5 KDa) to remove excess T7 peptide. After lyophilization, the final product of HAIYPRH-PEG2000-DSPE was obtained. The structure was characterized by NMR, see figure 1 , where A is the NMR spectrum of Mal-PEG2000-DSPE, B is the NMR spectrum of HAIYPRH-...
Embodiment 2
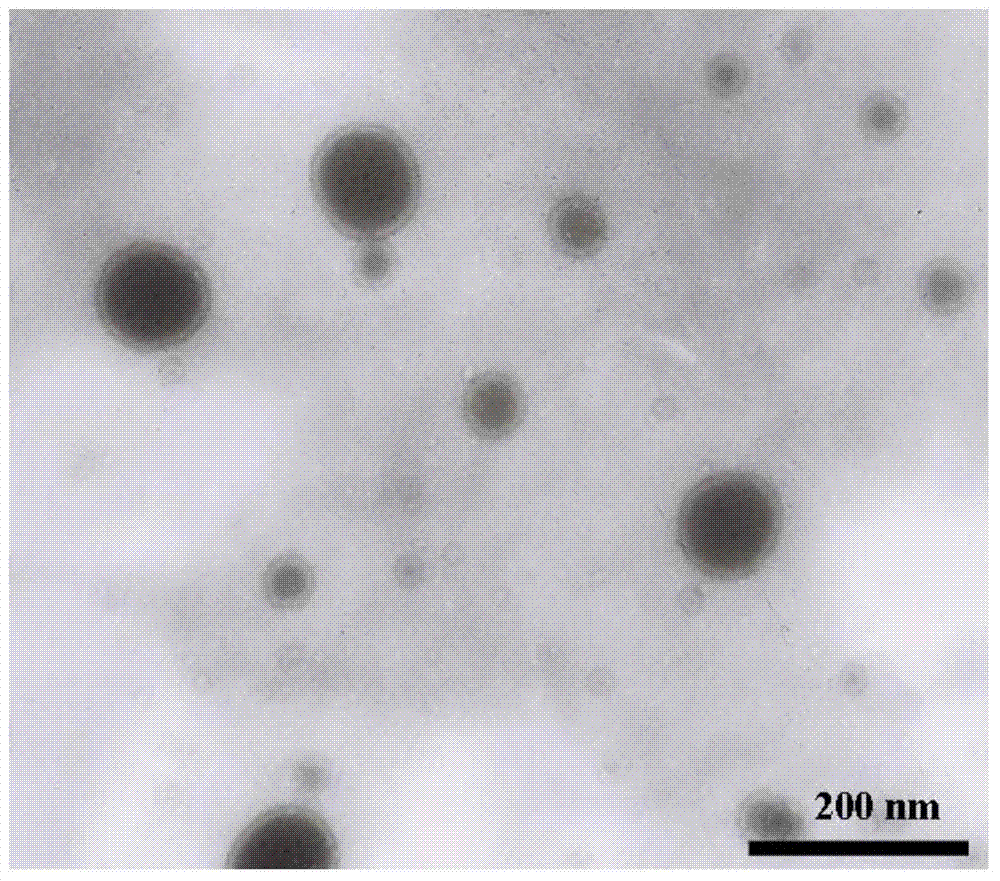
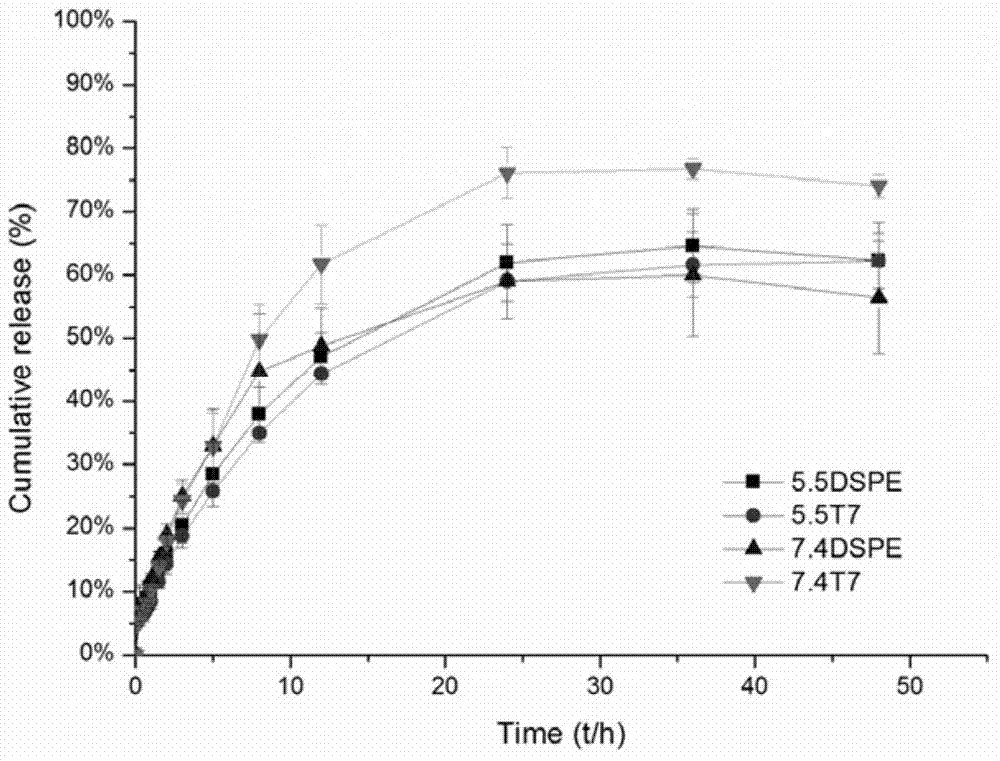
[0040] Preparation and characterization of T7 peptide-modified ZL006 long-circulating liposomes (HAIYPRH-long-circulating liposome-ZL006)
[0041] Prepared by ethanol injection method, dissolve soybean lecithin, cholesterol, methoxy-polyethylene glycol 2000-distearoylphosphatidylethanolamine (mPEG2000-DSPE), HAIYPRH-PEG2000-DSPE and ZL006 in 7.5ml ethanol, fully Vortex mixing, wherein the quality of soybean lecithin is 80 mg, the molar ratio of soybean lecithin / cholesterol / mPEG2000-DSPE / HAIYPRH-PEG2000-DSPE is 20:5:2:1, and the mass ratio of soybean lecithin / ZL006 is 20:5 . Then, the uniformly mixed solution was slowly dropped into 25 ml of pH5.5 phosphate buffer solution at 50° C. and a rotating speed of 350 rpm. After the dropwise addition was completed, keep at 50°C and continue to stir for 15 minutes. Subsequently, evaporate in a rotary evaporator for 5 min in a water bath at 60 °C (without degassing). After rotary evaporation, use an ultrasonic cell pulverizer to reduc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com