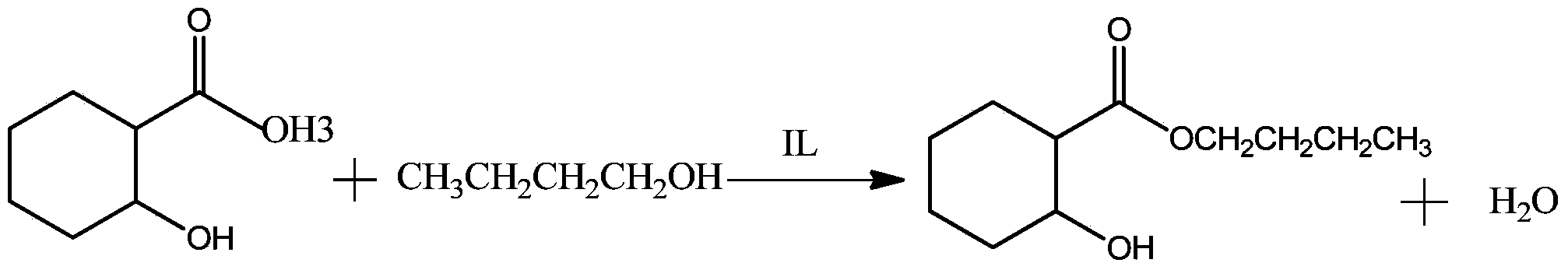Method for catalyzed synthesis of n-butyl salicylate and tributyl citrate by di-sec-butylamine acidic ionic liquids
A technology of acidic ionic liquid and n-butyl salicylate, which is applied in the field of esterification reaction and acidic ionic liquid of bis-secondary amine salts, can solve the problems of small market supply and high price of imidazole, and solve the problems of recycling, catalysts, etc. The effect of low dosage and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] When HY acid is sulfuric acid.
[0031] Add 7.10ml of 1,4-dibromobutane into a 250ml three-neck flask with a mechanical stirring device, add 80ml of acetonitrile as a solvent, and add 15.06ml of homopiperidine dropwise to 1,4-dibromobutane in an ice-water bath at 0°C Stir vigorously in the bromobutane solution at the same time, reflux in a water bath at 70°C under nitrogen protection for 24h; filter the obtained solid, wash with acetone 3 times and filter again, then dry at 1.33Pa at 70°C for 5h to obtain milky white needle-like crystals, which are bis-secondary amine salts. Then take 3.9g of bis-secondary amine salt and add it to a 250ml three-necked flask, add 80ml of dichloromethane to make it fully dissolved, then slowly add 1ml of 97% sulfuric acid according to the ratio of the amount of substances to 1:2, blow nitrogen, and reflux in a water bath at 40°C After 48 hours, dichloromethane was removed by rotary evaporation, washed twice with anhydrous ether and then r...
Embodiment 2
[0033] When the HY acid is p-benzenesulfonic acid.
[0034] Add 7.10ml of 1,4-dibromobutane into a 250ml three-necked flask with a mechanical stirring device, add 80ml of acetonitrile as a solvent, and add 15.06ml of homopiperidine dropwise to 1,4- Stir vigorously in the dibromobutane solution at the same time, reflux in a water bath at 70°C under nitrogen protection for 2 hours; filter the obtained solid, wash with acetone 5 times and filter again, then dry at 1.33Pa at 50°C for 8 hours to obtain milky white needle-like crystals, which are bis-secondary amine salts . Then take 3.9g of bis-secondary amine salt and add it to a 250ml three-neck flask, add 80ml of dichloromethane to make it fully dissolved, then slowly add p-toluenesulfonic acid according to the ratio of the substance to 1:2, blow nitrogen, and reflux in a water bath at 20°C for 5h , rotary evaporated to remove dichloromethane, washed twice with anhydrous ether and rotary evaporated again, then dried at 50°C for...
Embodiment 3
[0036] Use the ionic liquid in embodiment 1 as catalyst
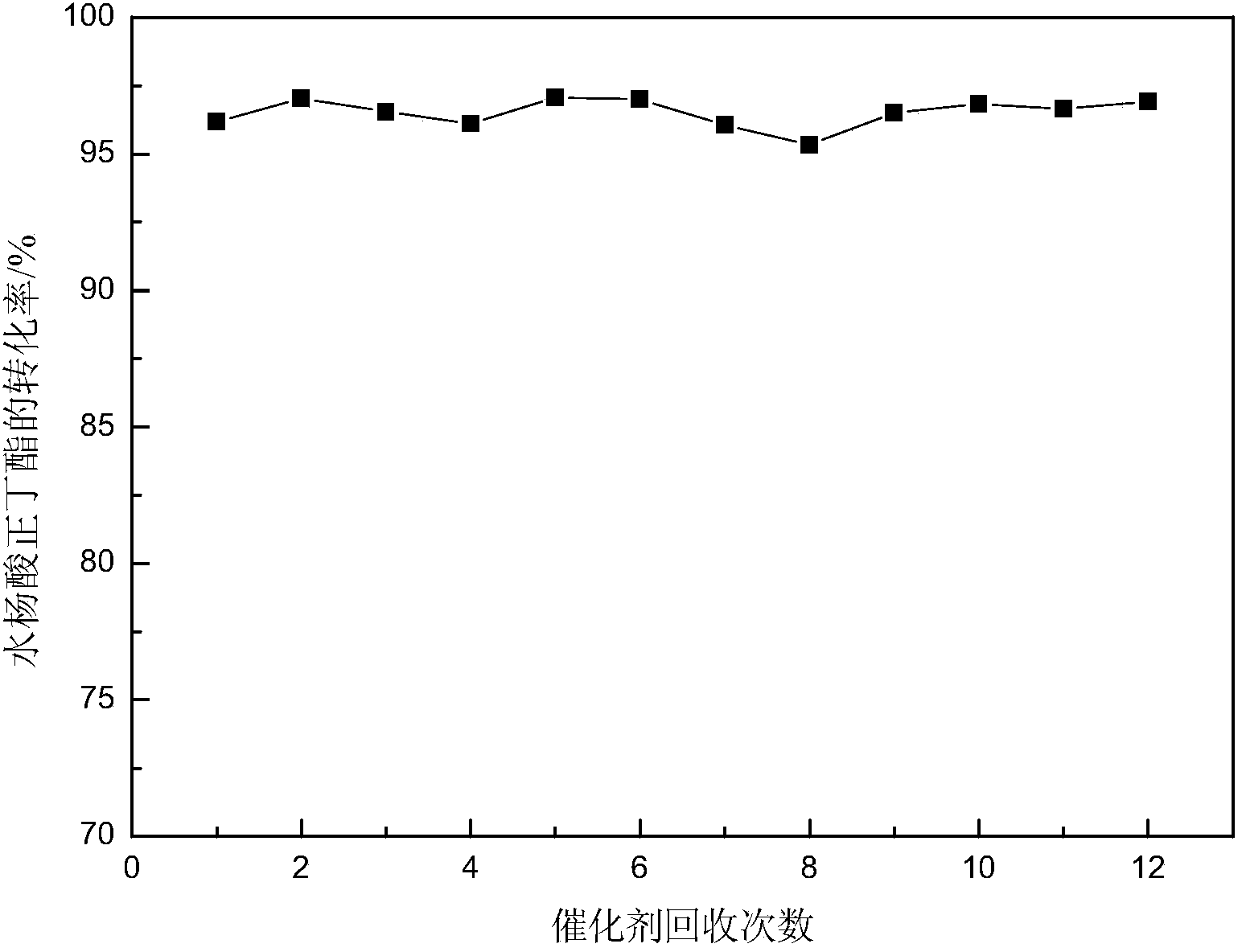
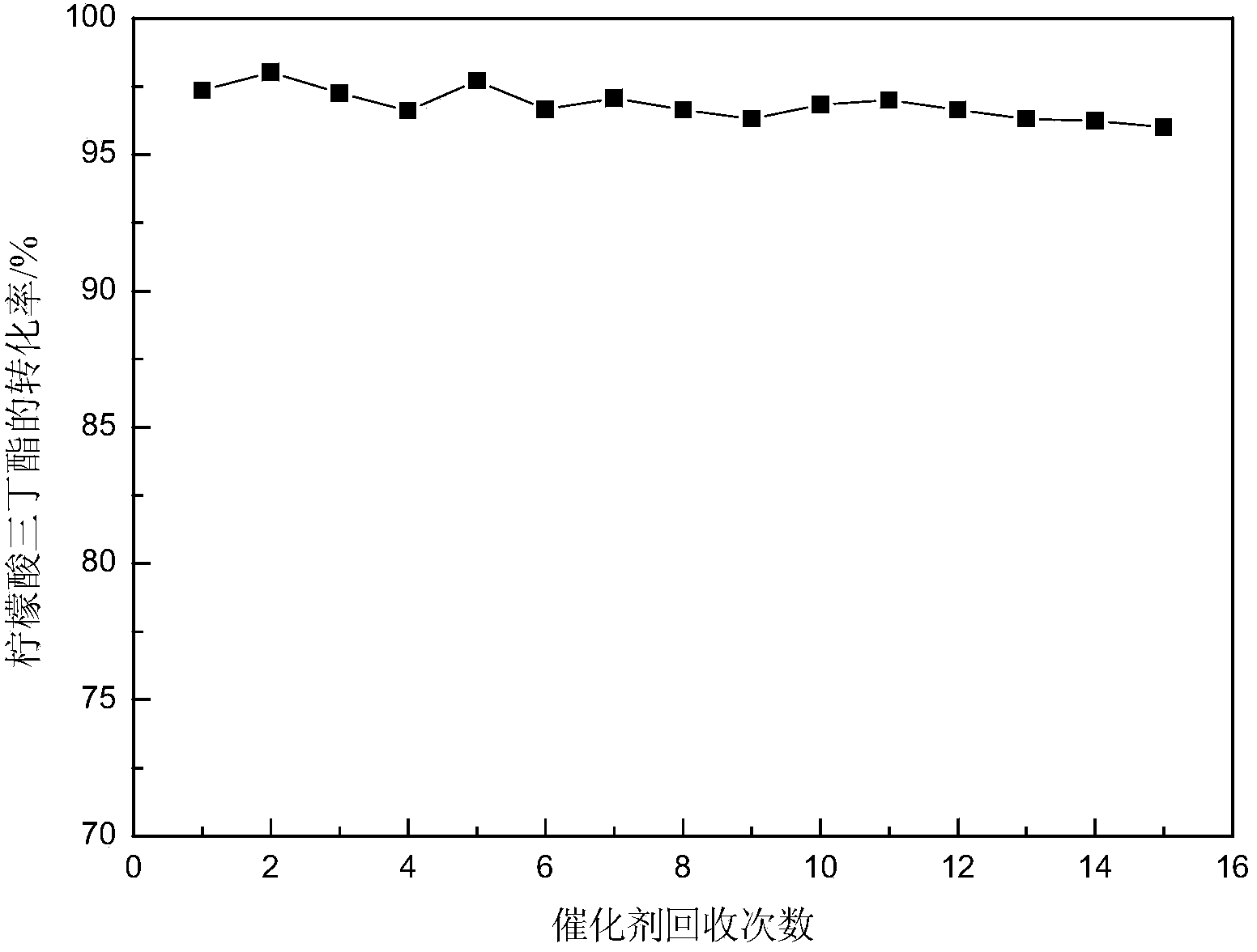
[0037]In a 250ml three-neck flask equipped with a water separator, an electric stirrer and a thermometer, react salicylic acid and n-butanol at a molar ratio of 1:1, and feed in a molar ratio of 1:1.5, and excess n-butanol is used as a water-carrying agent , Weigh 13.83g of salicylic acid and 13.73ml of n-butanol, first add the weighed salicylic acid and n-butanol into the three-necked flask, heat and stir at low temperature, and add 0.2766g of salicylic acid when it is completely dissolved The ionic liquid bis-(1-homopiperidine)butylene hydrogensulfate was used as a catalyst, and the addition amount was 2% of the mass of salicylic acid, and then the temperature was raised to 110°C, and the temperature was refluxed for 4 hours, and the water was divided in time during the reaction process. The conversion rate of salicylic acid can reach 94.38%, and the selectivity of n-butyl salicylate is 95.14%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com