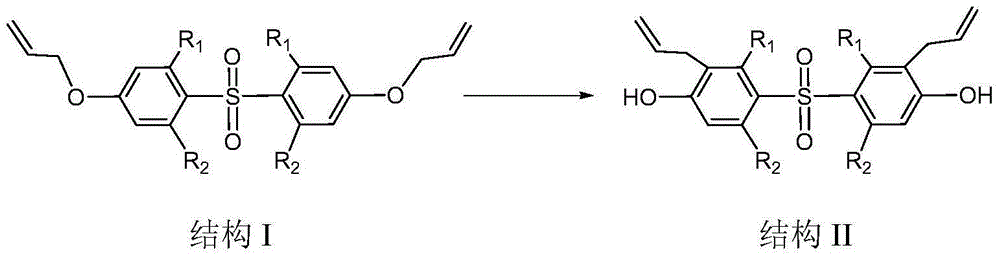
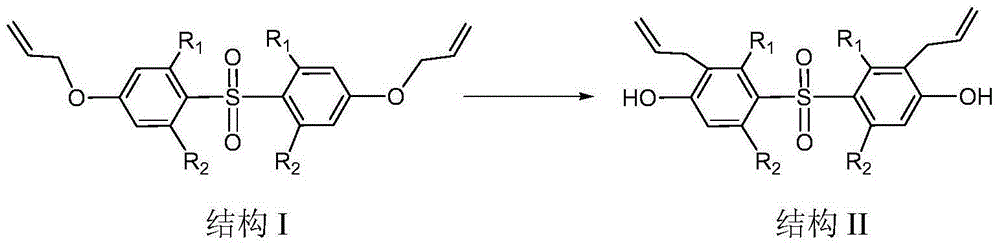
Preparation method of bis(3-allyl-4-hydroxyphenyl)sulfone and derivatives thereof
A technology of hydroxyphenyl and derivatives, which is applied in the field of preparation of bis(3-allyl-4-hydroxyphenyl) sulfone and its derivatives, can solve the problem of low regioselectivity, long reaction time, and difficulty in exporting products and other problems to achieve the effect of improving reaction efficiency, short reaction time and reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 52.8g of bis(4-allyloxyphenyl)sulfone, 120ml of m-toluene, 0.5g of graphene oxide and N,N-dimethyl Base aniline 0.05g, reacted at a temperature of 100°C for 60min. After the reaction, the products after the reaction were detected by HPLC, the content of the single rearrangement product was 0.33%, and the content of the double rearrangement product was 98%. The reaction solution was lowered to 60°C, 240g of 7% sodium hydroxide aqueous solution was added, graphene oxide was recovered by filtration, the filtrate obtained by filtration was separated to obtain an aqueous phase and an organic phase, and the obtained aqueous phase was adjusted to pH 2~2 with 60g concentrated hydrochloric acid. 3. Filtration, drying the obtained filter material at 105°C to obtain 51 g of bis(3-allyl-4-hydroxyphenyl)sulfone with a content of 99%, and a yield of 96.6%.
Embodiment 2
[0021] Add 52.8g of bis(4-allyloxyphenyl)sulfone, 60ml of m-toluene, 0.05g of graphene oxide and N,N-dimethyl 0.01 g of aniline, reacted for 40 min under the condition of 200° C., and the reaction ended. The content of the reactant was detected by HPLC, the content of the single rearrangement product was 0.40%, and the content of the double rearrangement product was 96%. The post-reaction treatment was the same as in Example 1, and 49 g of bis(3-allyl-4-hydroxyphenyl)sulfone with a content of 97.5% was obtained, with a yield of 92.8%.
Embodiment 3
[0023] Add 52.8g of bis(4-allyloxyphenyl)sulfone, 90ml of diphenyl ether, 0.2g of graphene oxide and N,N-dimethylaniline into the reactor with reflux condenser, stirrer and thermometer 0.03g, reacted at a temperature of 180°C for 50 minutes, and the reaction ended. The content of the reactant was detected by HPLC, the content of the single rearrangement product was 0.35%, and the content of the double rearrangement product was 97%. The post-reaction treatment was the same as in Example 1, and 50.2 g of bis(3-allyl-4-hydroxyphenyl)sulfone with a content of 98.2% was obtained, with a yield of 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com