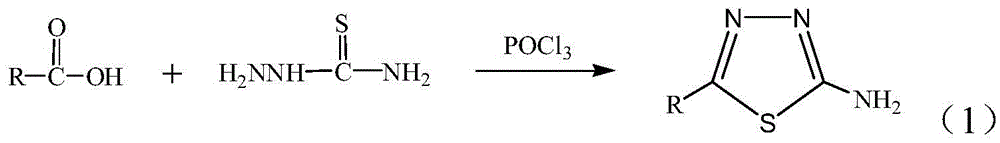A kind of method for preparing 2-amino-5-alkyl-1,3,4-thiadiazole
A thiadiazole and alkyl technology, which is applied in the field of preparing 2-amino-5-alkyl-1,3,4-thiadiazole, can solve the problems of high equipment requirements, long reaction time, complicated operation, etc. The effect of lowering the reaction temperature, convenience and ease of operation, and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
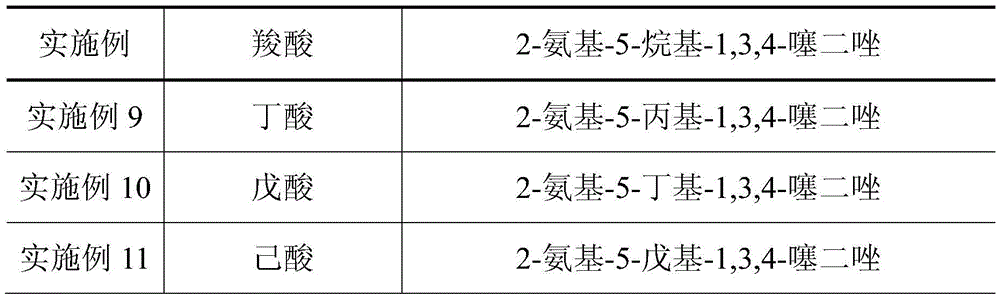
Embodiment 1
[0025] 1) Add 0.05mol thiosemicarbazide, 0.055mol acetic acid, 0.055mol phosphorus oxychloride and 0.25mol silica gel to a dry mortar, and grind for 10 minutes at room temperature. At this time, TLC monitoring shows that the raw material point of thiosemicarbazide disappears, indicating that the raw material Complete reaction, then leave standstill 30min, get crude product; Wherein the developer of TLC is the mixed solution of ethyl acetate and sherwood oil that volume ratio is 1:3;
[0026] 2) Move the crude product into a beaker, add a sodium carbonate solution with a mass concentration of 5% to the crude product until the pH value of the resulting mixed solution is 8, then filter the mixed solution with suction, and filter the obtained filter cake with solvent N , After dissolving N-dimethylformamide (DMF), continue suction filtration to remove silica gel, and then concentrate the final filtrate under reduced pressure to remove the solvent, then wash the product obtained by ...
Embodiment 2
[0029] 1) Add 0.05mol thiosemicarbazide, 0.055mol propionic acid, 0.055mol phosphorus oxychloride and 0.25mol silica gel to a dry mortar, and grind for 10 minutes at room temperature. At this time, TLC monitoring shows that the raw material point of thiosemicarbazide disappears, indicating The raw materials were completely reacted, and then left to stand for 30 minutes to obtain a crude product; wherein the TLC developer was a mixed solution of ethyl acetate and petroleum ether with a volume ratio of 1:3;
[0030] 2) Move the crude product into a beaker, add a sodium carbonate solution with a mass concentration of 5% to the crude product until the pH of the resulting mixture is 8, then filter the mixture with suction, and filter the obtained filter cake with solvent DMF Continue suction filtration after dissolution to remove silica gel, then concentrate the final filtrate under reduced pressure to remove the solvent, then wash the product obtained by concentration under reduced...
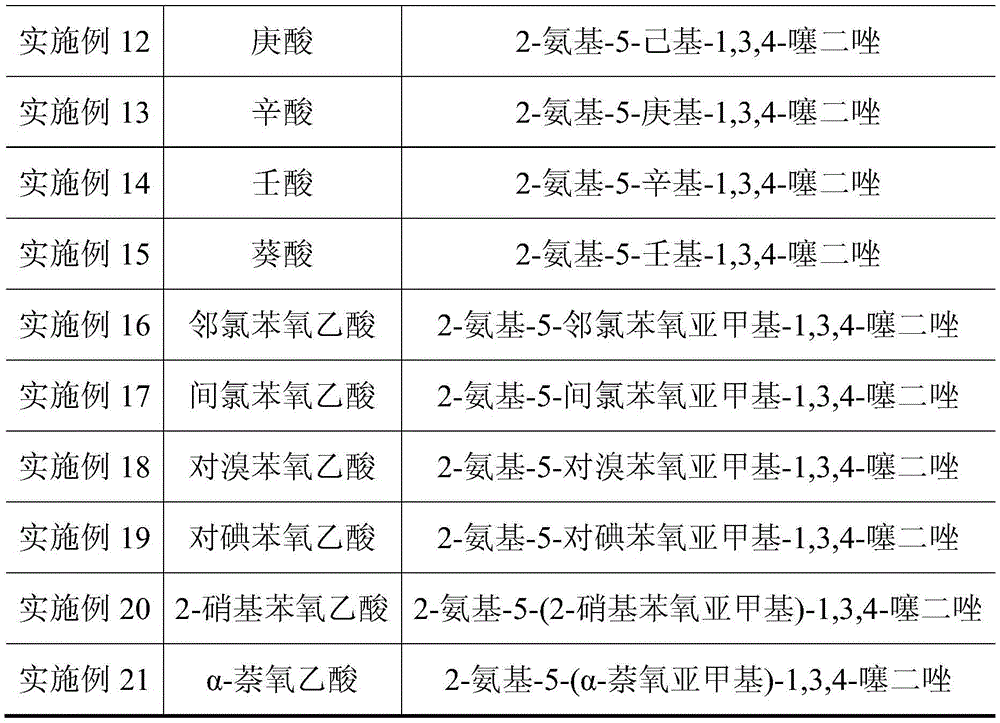
Embodiment 3
[0033] 1) Add 0.05mol thiosemicarbazide, 0.055mol phenoxyacetic acid, 0.055mol phosphorus oxychloride and 0.25mol silica gel to a dry mortar, and grind for 10min at room temperature. At this time, TLC monitoring shows that the raw material point of thiosemicarbazide disappears. Indicates that the raw materials are completely reacted, and then left to stand for 30 minutes to obtain a crude product; wherein the developer of TLC is a mixed solution of ethyl acetate and petroleum ether with a volume ratio of 1:3;
[0034]2) Move the crude product into a beaker, add a sodium carbonate solution with a mass concentration of 5% to the crude product until the pH of the resulting mixture is 8, then filter the mixture with suction, and filter the obtained filter cake with solvent DMF After dissolving, continue suction filtration to remove silica gel, then concentrate the final filtrate under reduced pressure, remove the solvent, then wash the product obtained by concentration under reduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com