Synthesis method for ectropis oblique sex pheromone
A synthesis method and pheromone technology, which are applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, catalytic reactions, etc., can solve the problems of less research on epoxidation reactions, and achieve simple and easy control. , The effect of less by-products and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
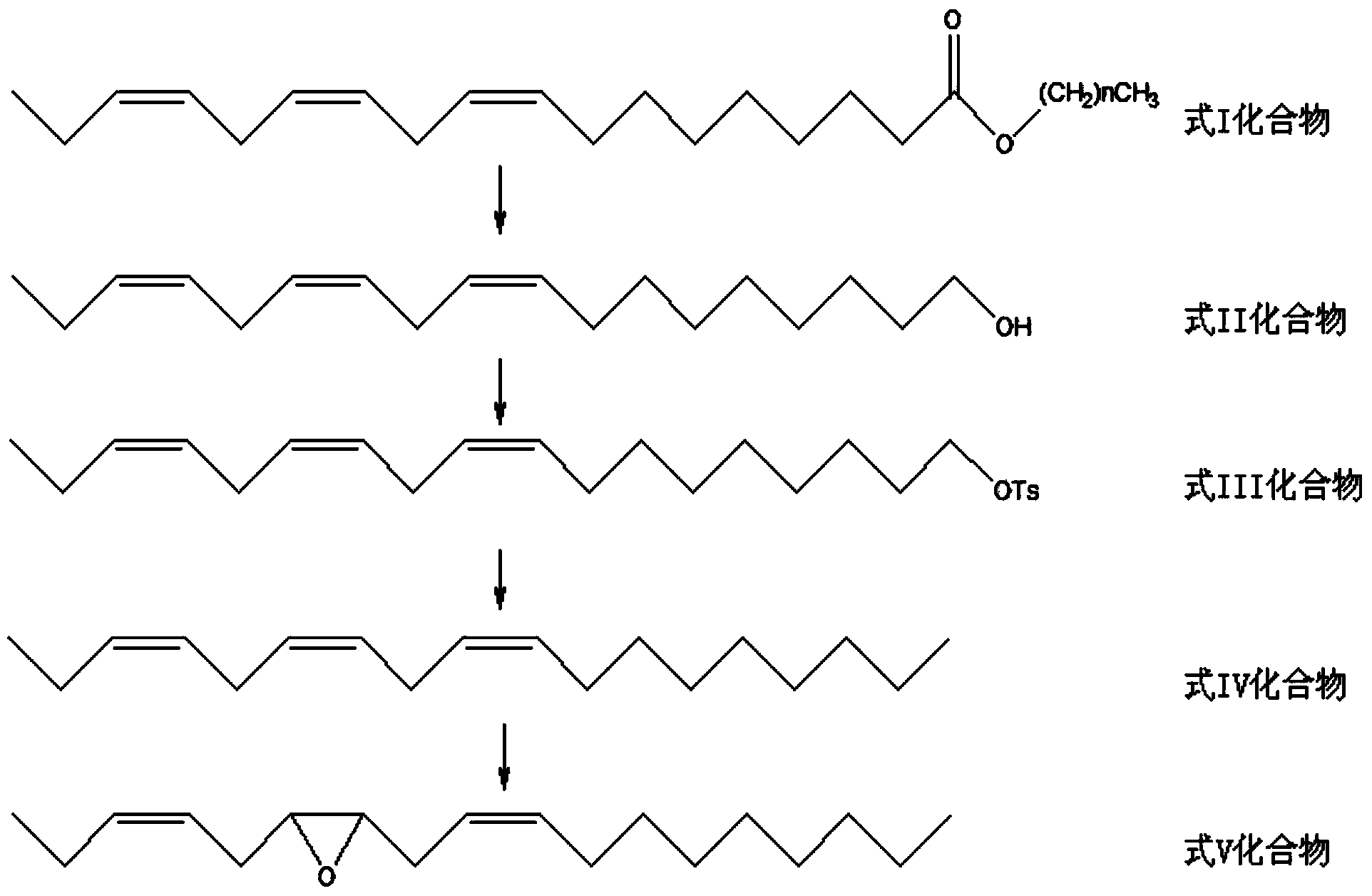
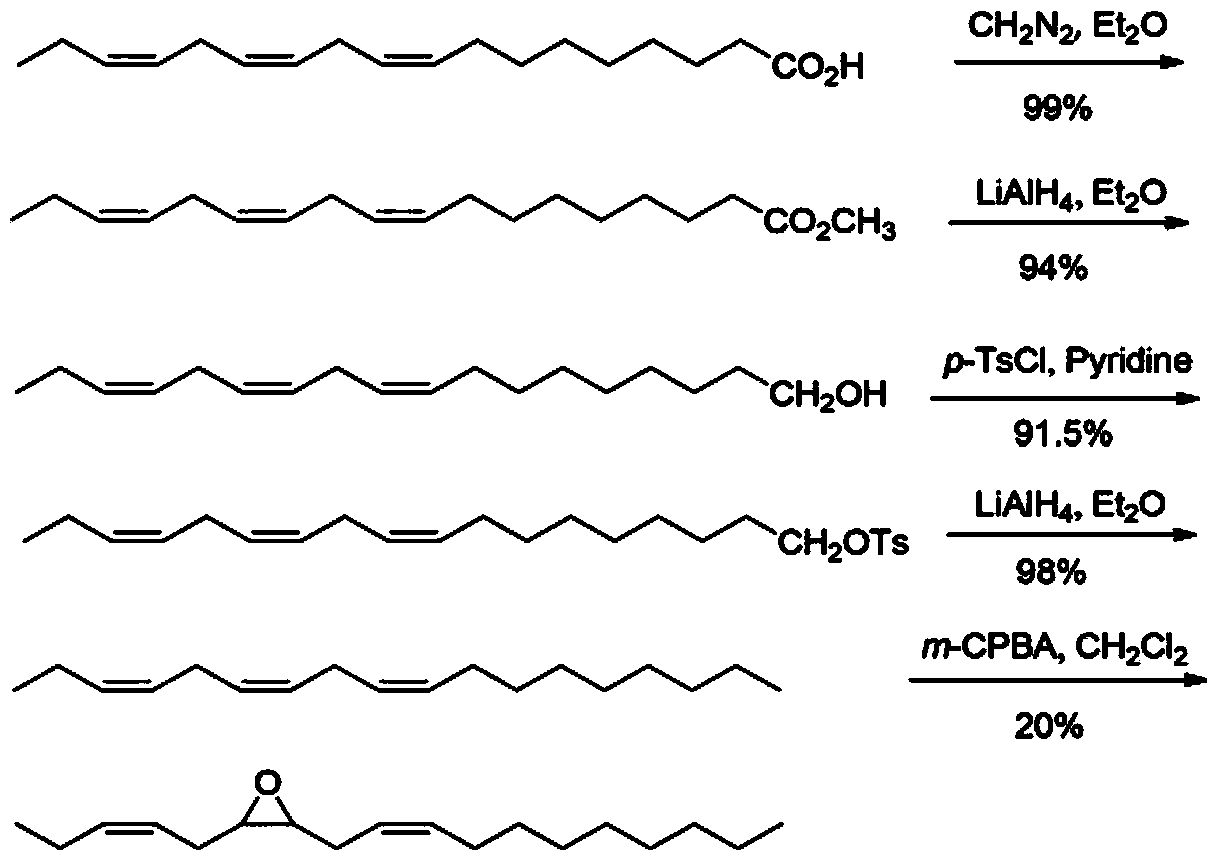
[0042] For the synthetic route map of tea geometrid sex pheromone, please refer to figure 1 , including the following steps:
[0043] Step 1. the synthesis of linoleyl alcohol
[0044] Under stirring and ice-bath conditions, slowly dropwise add 7g of methyl linolenate in 140ml of tetrahydrofuran solution to 2.8g of lithium aluminum tetrahydride in 70ml of tetrahydrofuran suspension, continue stirring at 10°C and monitor the progress of the reaction with a thin-layer plate. After 2 hours, the reaction is complete. Pour the reaction solution into the mixed system of ethyl acetate and anhydrous sodium sulfate, add 1ml of water under stirring conditions, and decompose the excess aluminum tetrahydrogen lithium; after the solution becomes clear, pour the ethyl acetate layer out, and the solvent was spin-dried to obtain linoleyl alcohol, which was directly put into the next reaction. The structural formula of linoleyl alcohol is:
[0045]
[0046] Step 2., the synthesis of lino...
Embodiment 2
[0067] Step 1. the synthesis of linoleyl alcohol
[0068] Under stirring and ice-bath conditions, slowly drop 6.5g of methyl linolenate in 135ml of tetrahydrofuran solution into 2.2g of lithium aluminum hydride in 65ml of tetrahydrofuran suspension, continue stirring at 12°C and monitor the progress of the reaction with a thin-layer plate . After 3 hours, the reaction is complete. Pour the reaction solution into the mixed system of ethyl acetate and anhydrous sodium sulfate, add 2ml of water under stirring conditions, and decompose the excess aluminum tetrahydrogen lithium; after the solution becomes clear, pour the ethyl acetate layer out, and the solvent was spin-dried to obtain linoleyl alcohol, which was directly put into the next reaction.
[0069] Step 2., the synthesis of linoleyl p-toluenesulfonate
[0070] Under stirring and cooling in an ice bath, slowly add 4.5 g of p-toluenesulfonyl chloride in 85 ml of dichloromethane solution dropwise to 5.5 g of linoleyl alcoh...
Embodiment 3
[0076] Step 1. the synthesis of linoleyl alcohol
[0077] Under stirring and ice-bath conditions, slowly dropwise add 7.5g of methyl linolenate in 145ml of tetrahydrofuran solution to 3g of lithium aluminum hydride in 75ml of tetrahydrofuran suspension, continue stirring at 15°C and monitor the progress of the reaction with a thin-layer plate. After 5 hours, the reaction is complete. Pour the reaction solution into the mixed system of ethyl acetate and anhydrous sodium sulfate, add 3ml of water under stirring conditions, and decompose the excess lithium tetrahydrogen aluminum; after the solution becomes clear, pour the ethyl acetate layer out, and the solvent was spin-dried to obtain linoleyl alcohol, which was directly put into the next reaction.
[0078] Step 2., the synthesis of linoleyl p-toluenesulfonate
[0079]Under stirring and cooling in an ice bath, slowly add 5.5 g of p-toluenesulfonyl chloride in 95 ml of dichloromethane solution dropwise to 6.5 g of linoleyl alco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com