Lyophilized stabilizer composition of human plasma protein C and use of composition
A technology of human plasma protein and freeze-drying stabilizer, which is applied in the field of plasma products, can solve the problems of damage to the target protein activity, protein structure denaturation, protein C activity reduction, etc., and achieves small loss of activity, high recovery rate, and improved appearance and molding. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
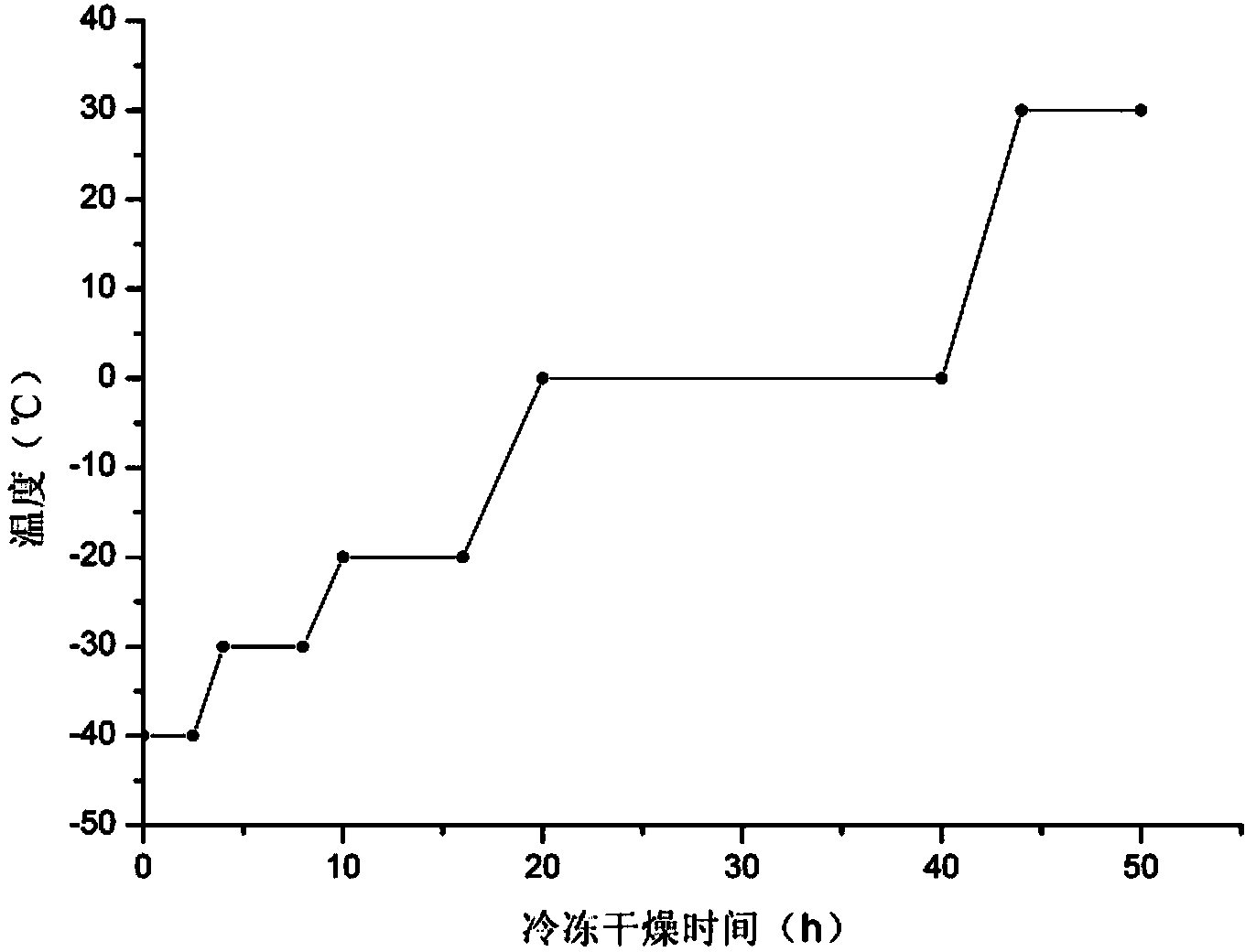
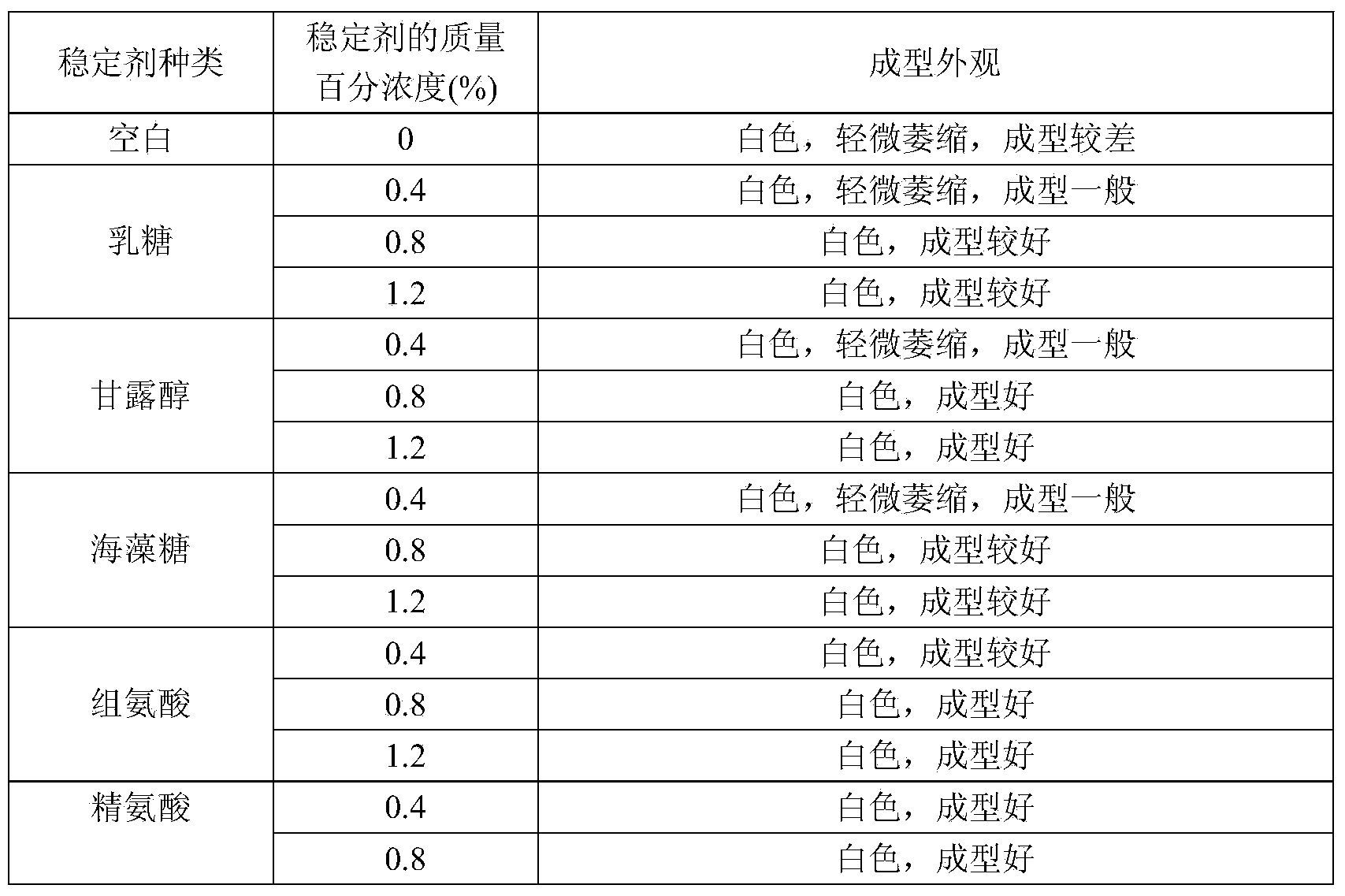
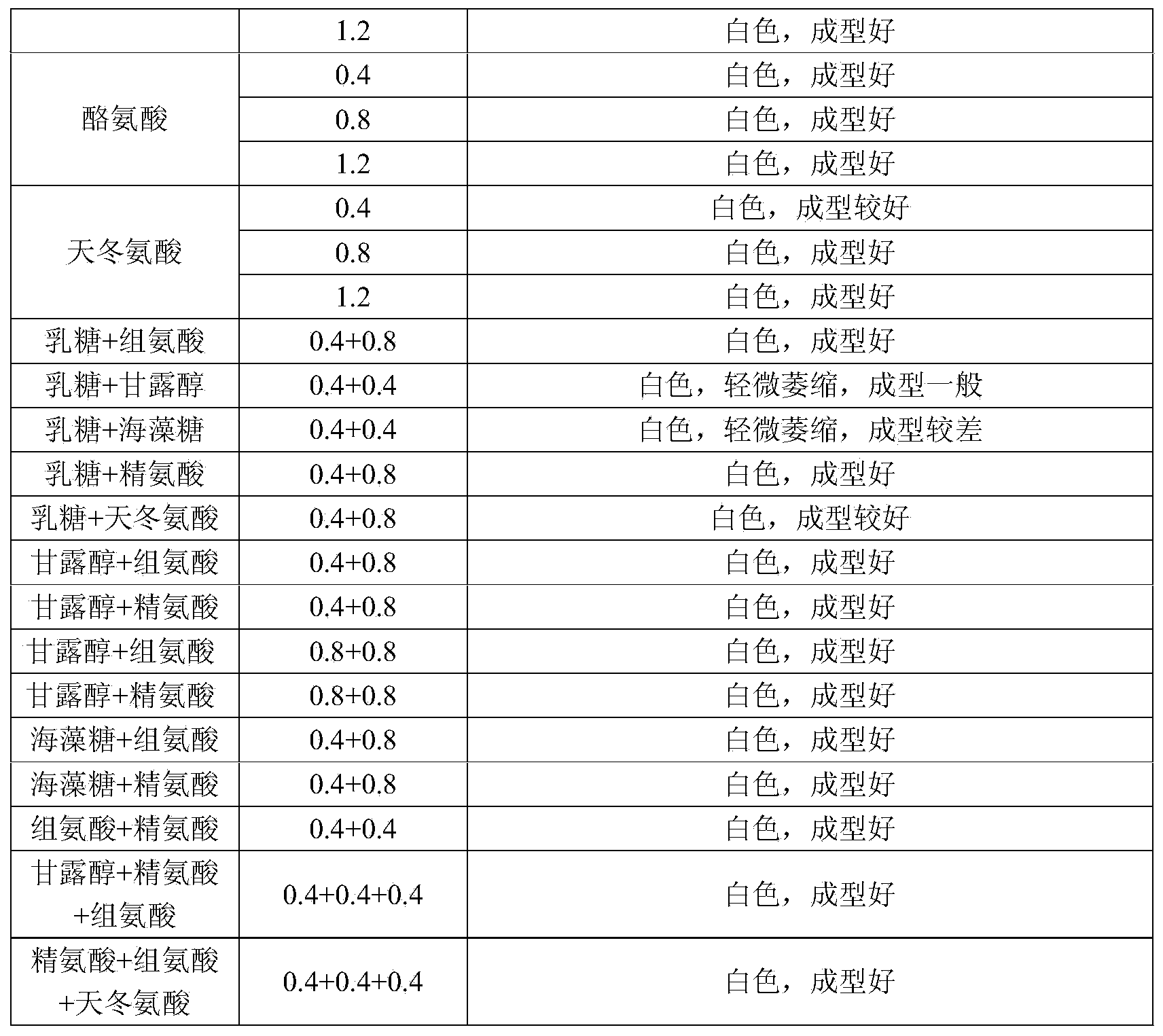
Image
Examples
Embodiment 1
[0053] Embodiment 1 Preparation of human plasma protein C preparation of the present invention
[0054] 1. Preparation method
[0055] Ⅰ. Preparation of Human Protein C Concentrate
[0056] (1) Preparation of human cryoprecipitate plasma
[0057] About 1000L of human fresh frozen plasma that meets the requirements of the Pharmacopoeia (weight is about 1000kg), after disinfecting the plasma bag with alcohol, melt the plasma with water for injection at 0-5°C. The cryoprecipitated plasma was removed by centrifugation for later use.
[0058] (2) Gel adsorption and purification
[0059] ①DEAE Sephadex A50 adsorption: fully swell 2kg of DEAE Sephadex A50 gel dry powder, balance with balance solution (0.015M sodium citrate, 0.08M sodium chloride, pH7.3) for 5 times, 60L each time, and then add step (1 ) and the cryoprecipitated plasma was stirred and adsorbed at 2-8°C for 30 minutes, the supernatant was put into the plasma tank, and the gel was collected.
[0060] ② Washing: was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com