Stellera chameajasme component film-coated tablet
A technology of Daphne chamaema chamaejasme and film-coated tablets, which can be applied to sugar-coated pills, drug combinations, medical preparations containing active ingredients, etc., and can solve the problems of poor stability, poor solubility in water, and poor stability of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
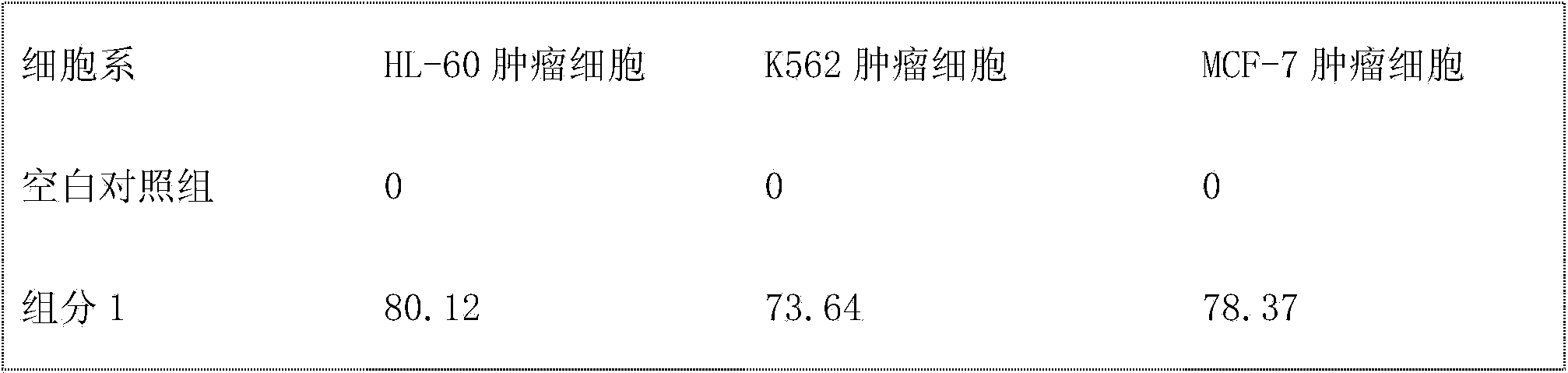
Embodiment 1
[0077] Preparation of Daphne chamaejasme Component 1:
[0078] A Daphne chamaejasme extract is characterized in that it is prepared by the following method:
[0079] Step 1. After pulverizing 1 kg of Rhizoma chamaejasma, add 10 L of acetone: ethanol = 2: 1 (v: v) mixed solution, heat and reflux for 1 hour, filter out the extract, and add 10 L of acetone: ethanol = 2: 1 ( The mixed solution of v: v) was heated to reflux for 1 hour, the extract was filtered out, and the filtrate was combined to obtain the extract;
[0080] Step 2, recover the solvent from the combined extract under reduced pressure, concentrate and dry to obtain 85g of dry extract, take 2kg of HPD100 type macroporous resin, mix the sample with the dry extract; first, use ethanol:ethyl acetate=1: 1 (v: v) 5 L was used as the mobile phase for elution, and then 95% ethanol (v: v) 5 L was used as the mobile phase to elute to obtain an eluent. After recovering the ethanol, the eluate was concentrated and dried to ob...
Embodiment 2
[0085] Tablet preparation
[0086] The components obtained in Example 1 are used as pharmaceutical active ingredients, added with pharmaceutical carriers, and prepared according to the conventional techniques of pharmacy, such as the following preparation method:
[0087] 10 mg of component 1, 200 mg of starch, 100 mg of powdered sugar, appropriate amount of magnesium stearate, using starch paste as a binder, mixing, granulating, air-drying, sizing, and tableting to obtain tablets.
[0088] Tablet stability test:
[0089] 100 mg of component 1 and the tablet prepared by the above method were placed in an incubator at 40° C., and observed after 3 months, the appearance and color did not change in any way.
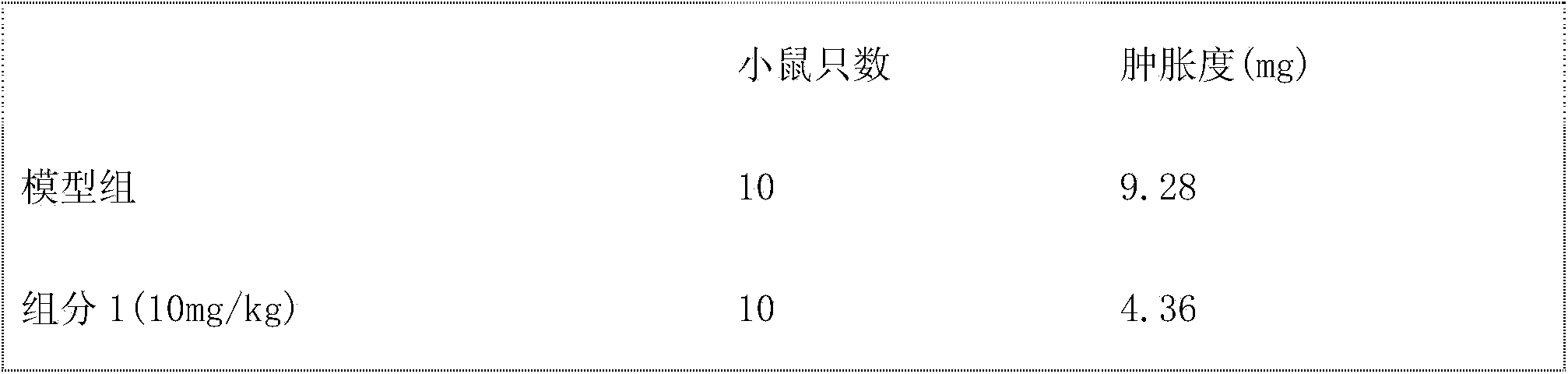
Embodiment 3
[0091] Preparation of sugar-coated tablets
[0092] The components obtained in Example 1 are used as pharmaceutical active ingredients, added with pharmaceutical carriers, and prepared according to the conventional techniques of pharmacy, such as the following preparation method:
[0093] 10mg of component 1, 200mg of starch, 100mg of powdered sugar, appropriate amount of magnesium stearate, using starch paste as a binder, mixing, granulating, air-drying, sizing, tableting to obtain tablets, and coating with syrup to obtain sugar coating piece.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com