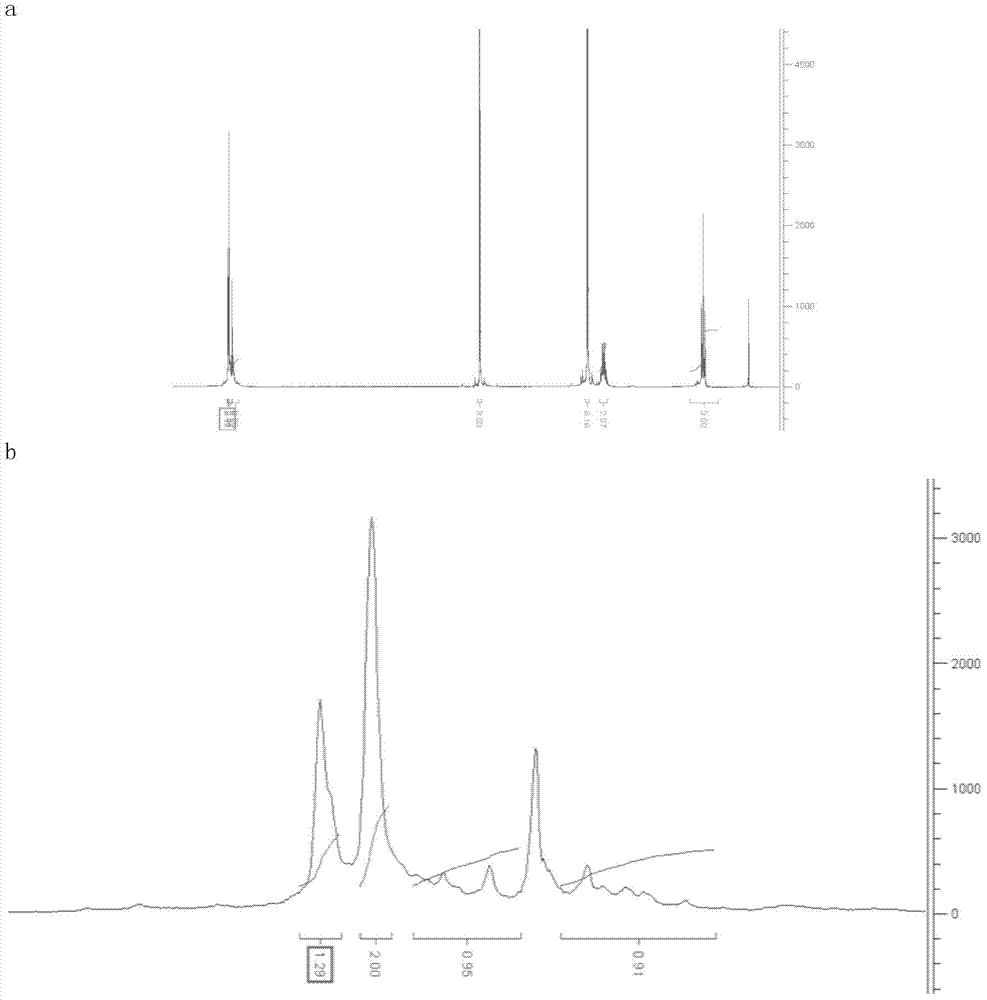
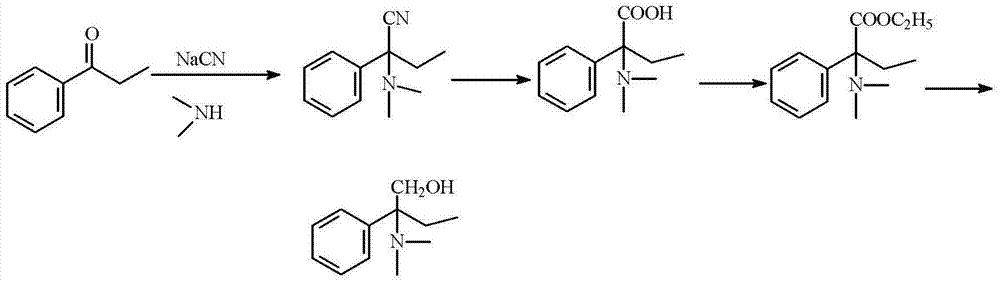
Method of preparing 2-(N, N- dimethylamino)-2 phenyl butanol
A technology of dimethylamino and phenylbutanol, applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc., can solve the problems of low total yield, long reaction steps, etc., and achieves less by-products, The effect of mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Preparation of 2-(N,N-dimethylamino)-2-phenylbutyronitrile: 100g propiophenone (0.75mol), 45g sodium cyanide (0.91mol), 337g40% dimethylamine methanol solution, 140g of water is put into a 1L autoclave, the mouth of the kettle is wiped clean, and the lid of the kettle is closed. After stirring for 30 minutes, the temperature began to rise, and the addition reaction was carried out at a pressure of 0.3 MPa and a temperature of 80° C. for 8 hours. After the reaction is over, turn on the cooling water to cool down to 30°C, open the pressure relief valve to release the pressure; open the autoclave, pour out the material, rinse it with water, add water to a total volume of 1200ml, cool down to 10°C with ice salt water, filter, and use Wash the filter cake with 100ml×2 water (the filtrate and washing liquid are treated with aqueous potassium permanganate solution), and dry the filter cake to obtain 153g of the product, with a content (HPLC) of 99.6%, and a yield of 86.4%; ...
Embodiment 2
[0048]1. Preparation of 2-(N,N-dimethylamino)-2-phenylbutyronitrile: 100g propiophenone (0.75mol), 42g sodium cyanide (0.85mol), 330g40% dimethylamine methanol solution, water 140g, put it into a 1L autoclave, wipe the mouth of the kettle clean, and close the lid of the kettle. After stirring for 30 minutes, the temperature began to rise, and the addition reaction was carried out at a pressure of 0.3 MPa and a temperature of 80° C. for 8 hours. After the reaction is over, turn on the cooling water to cool down to 30°C, open the pressure relief valve to release the pressure; open the autoclave, pour out the material, rinse it with water, add water to a total volume of 1200ml, cool down to 10°C with ice salt water, filter, and use Wash the filter cake with 100ml×2 water (the filtrate and washing liquid are treated with potassium permanganate aqueous solution), and dry the filter cake to obtain 149g of the product, with a content (HPLC) of 99.3%, and a yield of 84.1%.
[0049] 2...
Embodiment 3
[0058] 1. Preparation of 2-(N,N-dimethylamino)-2-phenylbutyronitrile: 100g propiophenone (0.75mol), 45g sodium cyanide (0.91mol), 337g40% dimethylamine methanol solution, 140g of water is put into a 1L autoclave, the mouth of the kettle is wiped clean, and the lid of the kettle is closed. After stirring for 30 minutes, the temperature was raised, and the addition reaction was carried out at a pressure of 0.3 MPa and a temperature of 60° C. for 8 hours. After the reaction is over, turn on the cooling water to cool down to 30°C, open the pressure relief valve to release the pressure; open the autoclave, pour out the material, rinse it with water, add water to a total volume of 1200ml, cool down to 10°C with ice salt water, filter, and use Wash the filter cake with 100ml×2 water (the filtrate and washing liquid are treated with potassium permanganate aqueous solution), and dry the filter cake to obtain 147g of the product, with a content (HPLC) of 99.1%, and a yield of 83%.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com