Recombinant polypeptide for treating tumors
A technology of recombinant polypeptides and antigenic peptides, applied in the field of tumor treatment, can solve the problems of weak antigenicity, insufficient recognition of immune cells, activation and killing, etc., and achieve the effect of simple production and preparation, low cost, and enhanced killing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
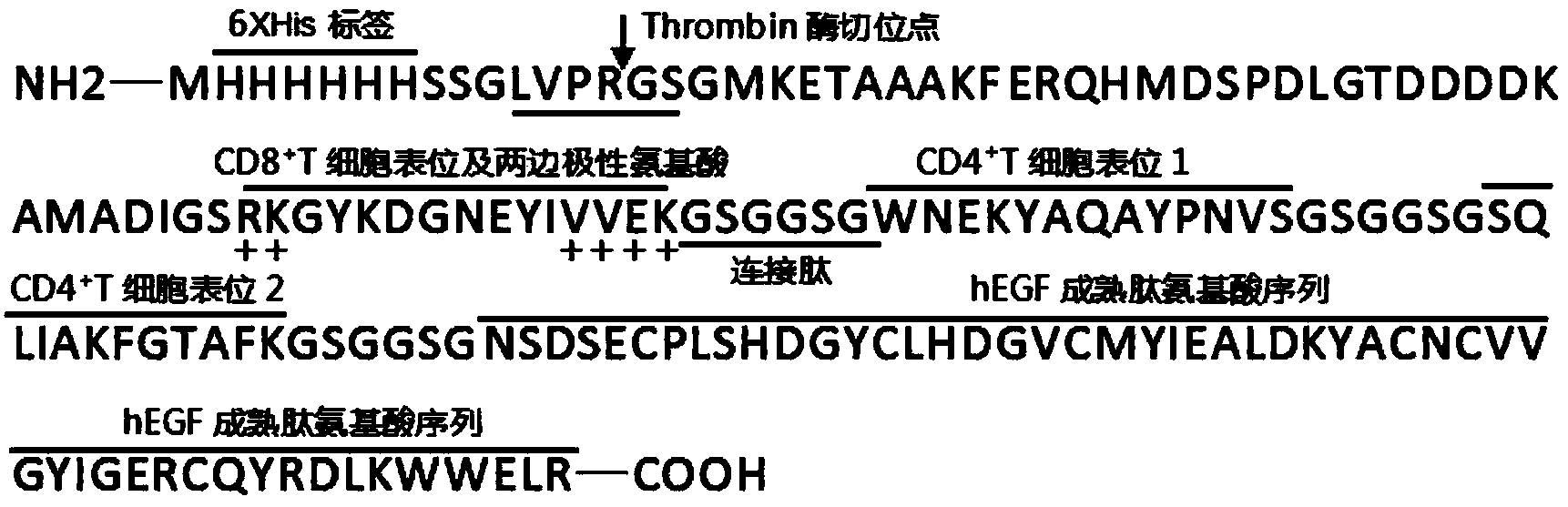
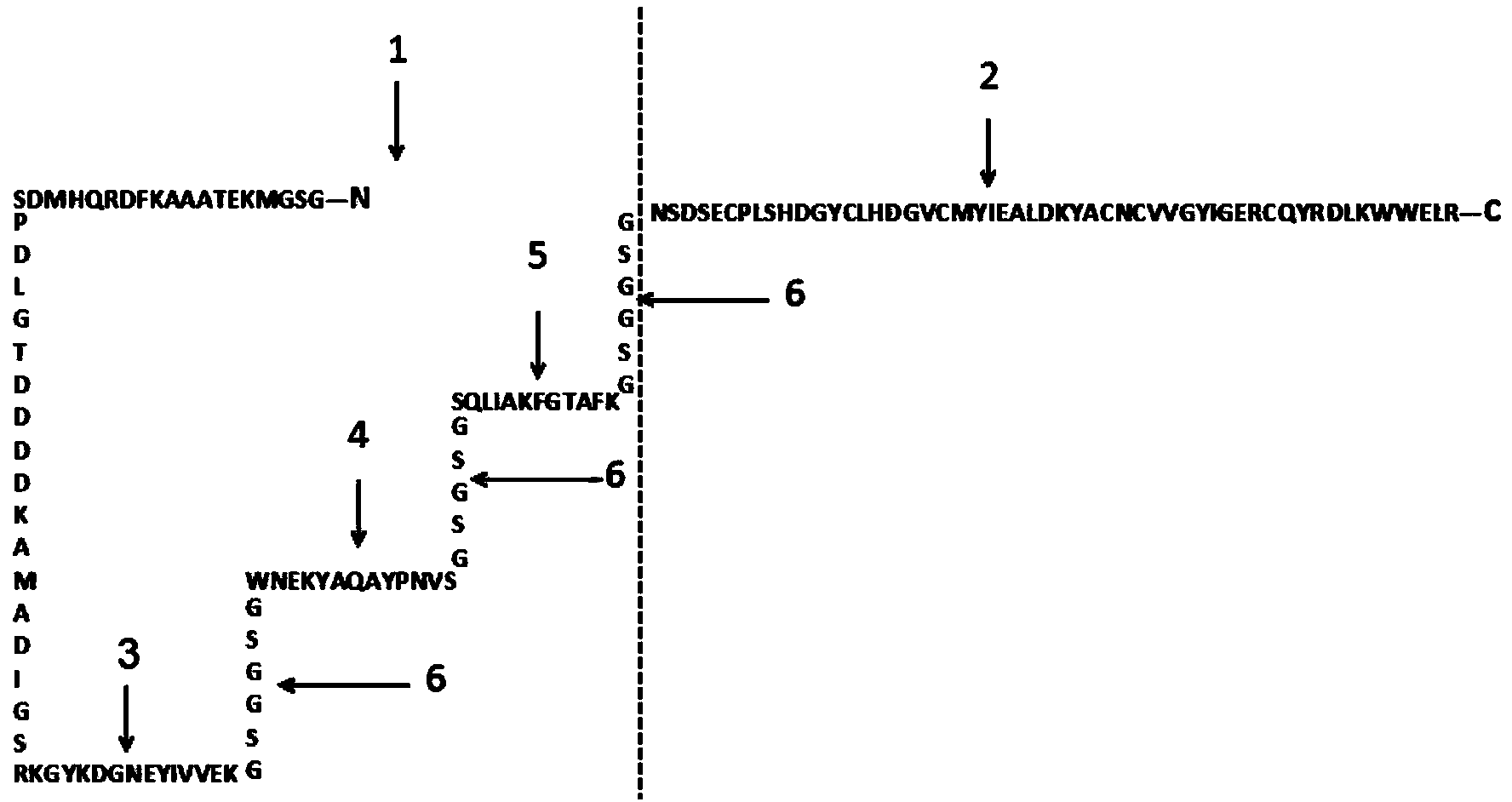
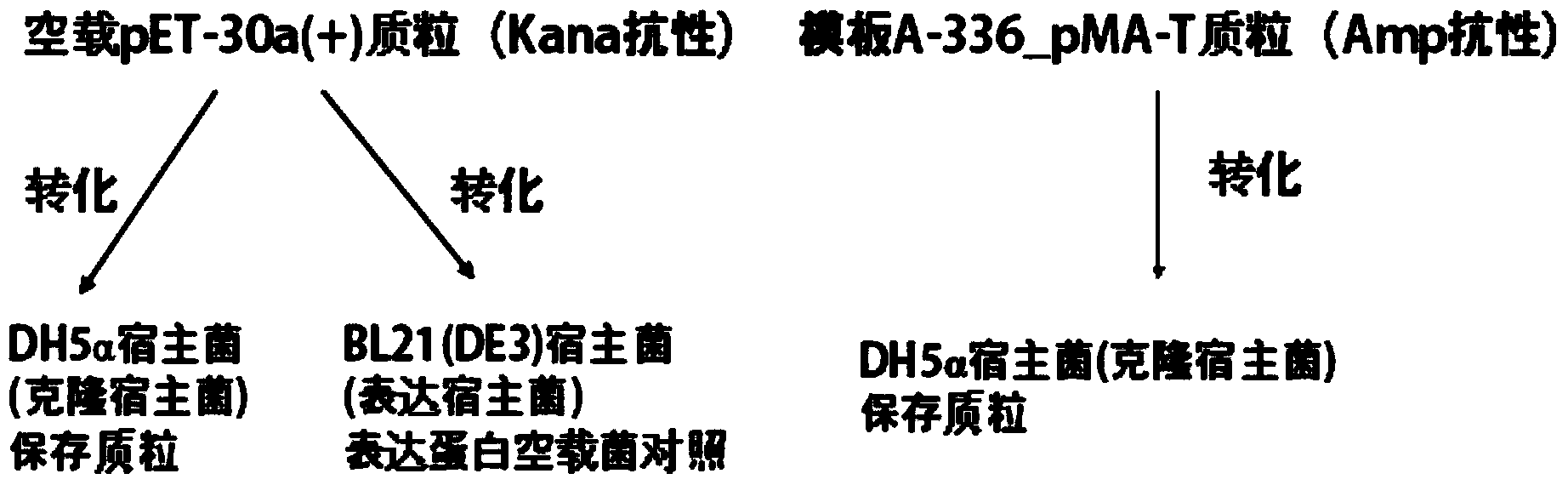
[0029] The 336bp DNA sequence was designed, including three dominant antigen peptide nucleotide sequences from LLO and hEGF nucleotide sequence, and the linking peptide nucleotide sequence between the coding sequences of different peptides. The designed sequence was artificially synthesized by chemical synthesis and constructed from the template plasmid: A-336_pMA-T (Amp + ). exist Figure 2A , the expression plasmid pET-30a(+) was extracted by alkaline lysis method, and 1 μg was added to 50 μl of competent cells DH5a and 50 μl of competent cells BL21(DE3) respectively. Heat shock at ℃ for 60 s, put on ice for 2 min, add 800 μl of LB medium respectively, set at 37 ℃ shaker at 200 rpm, shake for 90 min, centrifuge at 3000 rpm for 5 min in a centrifuge, discard the supernatant, leave 100 μl of medium, and resuspend the bacteria carefully After exposure, glass rods were used to spread on Kana-resistant plates. The plate was placed in a 37°C incubator and incubated upside down ...
Embodiment 2
[0031] like Figure 3A Induced expression of recombinant protein (pLLO-hEGF) as indicated. First, inoculate the correctly sequenced recombinant monoclonal bacterial solution into 5ml / support LB medium at a ratio of 1:100, add Kana antibiotics at a ratio of 1:1000, and shake at 37°C and 250rpm for culture. OD of the bacterial solution 600 At 0.6 to 0.8, take a 5ml strain of bacteria and use it as a small amount of recombinant protein to induce expression. The inducer IPTG was added to the bacterial solution to make the final concentration 1 mM, and the culture was continued at 37° C. and 250 rpm for 4 h. When the recombinant protein was induced and expressed at 37°C, it mainly existed in the form of inclusion bodies. Take 1ml of pre-induction and post-induction bacteria solution, centrifuge at 10,000 rpm for 1 min, add 100 μl of 1X SDS loading buffer, boil at 100 °C for 10 min, and centrifuge at 12,000 rpm for 5 min after cooling. Take the supernatant as the loading sample f...
Embodiment 3
[0033] exist Figures 4A-4F Among them, a recombinant protein (pLLO-hEGF) can target and bind to a variety of tumor cells. Cell immunochemical staining was used to analyze whether the recombinant protein could bind to tumor cells with high expression of EGFR. Preparation of cell slides of different tumor cells, including colorectal cancer cells HCT116 and HT29 ( Figure 4A and 4B ), lung cancer cells A549 and NCI-H157 ( Figure 4C and 4D ), breast cancer cells SK-BR-3 and MDA-MB-231 ( Figure 4E and 4F). The cell slides were washed 3 times with PBS, 2 min each time. 4% paraformaldehyde was fixed at room temperature for 30 min, and the slides were immersed in PBS with small forceps and washed three times for 5 min each time. Blocked with 3% BSA at room temperature for 30 min, washed twice with PBS for 5 min each time. Set up 3 groups: anti-EGFR Rabbit mAb group, namely positive control group; EGF control group; recombinant protein group. EGF and recombinant protein we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com