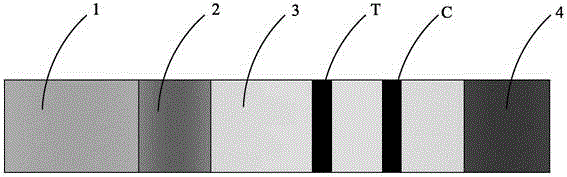
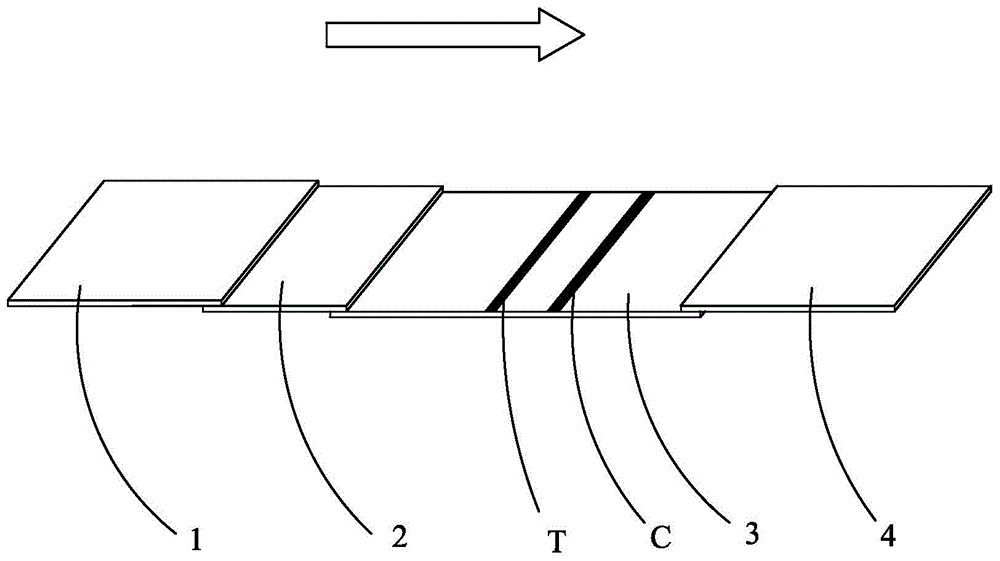
Human h-fabp colloidal gold detection test paper and preparation method thereof
A technology for detecting test strips and colloidal gold, applied in the field of in vitro diagnostic medical testing, can solve the problems of high price of end products, unfavorable POCT popularization and application, and high economic cost, and achieve the effect of shortening sample turnaround time, improving labeling effect, and promoting rapid release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of H-FABP colloidal gold detection test paper
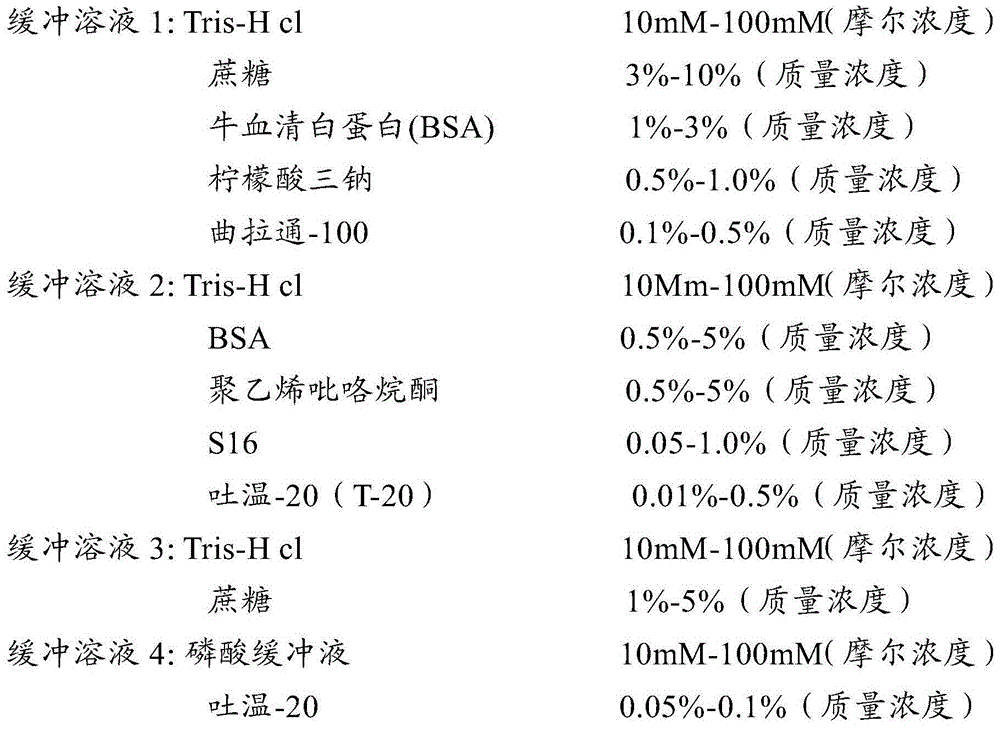
[0038] In this embodiment, the buffer system solution involved in the preparation of the detection test paper of the present invention is prepared according to the following formula:
[0039]
[0040]
[0041] The preparation method of H-FABP colloidal gold detection test paper comprises the steps:
[0042] Preparation of colloidal gold particles around 1.40nm:
[0043] (1) Add 200ml 0.01% chloroauric acid solution in a 250ml Erlenmeyer flask, use a magnetic heating stirrer at 180r / min to stir and heat to boiling;
[0044] (2) keep boiling for 1min;
[0045] (3) Increase the rotating speed to 350r / min, and quickly add 2ml of 2% trisodium citrate solution to the side of the vortex;
[0046] (4) Continue to boil for 5 minutes, stop heating, continue stirring and cooling;
[0047] (5) Dilute to 200ml with double distilled water;
[0048] (6) Measured by a spectrophotometer, the highest...
Embodiment 2
[0066] Example 2: Detection time and sensitivity
[0067] 1. Prepare three different buffer solutions 1: ①, ② and ③, ① add 0.05% PEG-20000 in mass concentration, ② do not add PEG-20000, ③ add 0.5% trisodium citrate to prepare three different gold Label conjugate pad;
[0068] 2. Use high, medium and low-value quality control products with three different concentrations to test three different detection test papers, among which the concentrations of high, medium and low-value quality control levels are 120ng / ml, 30ng / ml, and 6ng / ml respectively , the results are shown in Table 1.
[0069] 3. Utilize the test paper KHB prepared according to the preparation method of Example 1 and the existing product A with a good reputation on the market to compare the detection time, and the results are shown in Table 2.
[0070] 4. Utilize a series of H-FABP quality control products diluted by gradient, detect respectively with the detection test paper of the present invention, the result s...
Embodiment 3
[0080] Embodiment 3: detection test paper analytical performance - specificity
[0081] 1. Prepare two different sample pad treatment solutions, namely buffer solution 2: ① without S16 and Tween-20; ② with 0.5% S16 and 0.05% Tween-20. Two different test papers ① and ② were prepared by treating the sample pad with the above two different treatment solutions.
[0082]2. Compared with the existing marketed product A, a representative viscous serum sample (moderately positive) was used for detection, and the results are shown in Table 3.
[0083] Table 3 Effect of viscous samples on specificity
[0084]
5min
10min
15min
Test strip①
+
++
+++
Test paper②
+
++
++
Existing product A
+
++
+++
[0085] Note: "-" means negative, "±" means gray area, "+" means weak positive, "++" means moderate positive, "+++" means strong positive.
[0086] 3. No cross-interference phenomenon was found in the test p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com