Nonlinear polymer having doxorubicin structure, and preparation method and application thereof
A doxorubicin and polymer technology is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, organic active ingredients, etc. It can solve problems such as low solubility, short half-life, and high toxicity, and achieve Easy to dissolve, excellent effect, half-life extended effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1) A mixture of 80 g of sebacic acid in 800 ml of acetic anhydride was refluxed to form acetyl-sebacic acid;
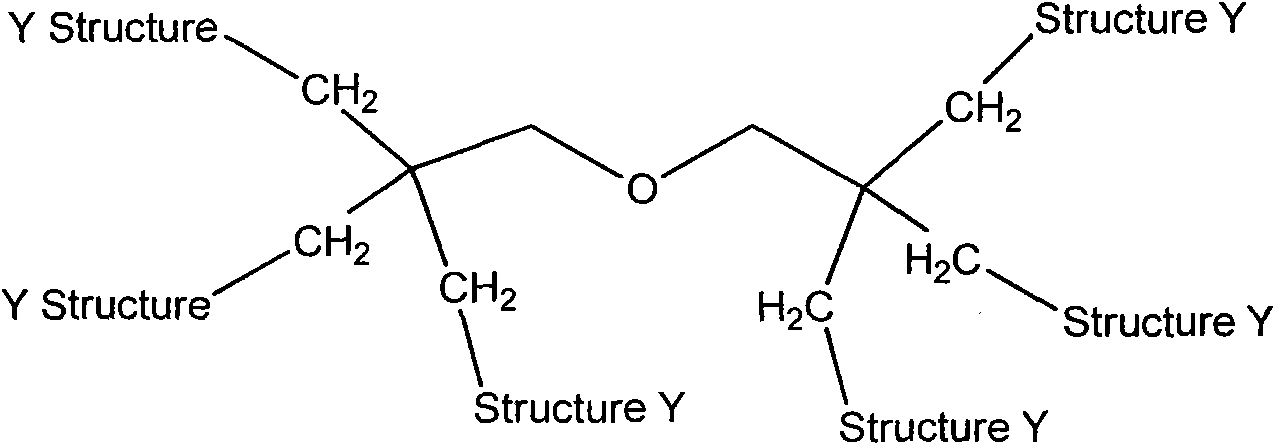
[0052] 2) Mix the 1st) step with the 6-arm polyethylene glycol 14g product terminated with hydroxyl and put it into a flask, and at 180 ° C under reduced pressure solution polymerization reaction 1 hour; ether washed and dried;
[0053] 3) Put 120 mg of doxorubicin and 800 mg of polymer from step 2 into 8 ml of dimethyl sulfoxide and 12 ml of dichloromethane solution for 48 hours; ultrasonicate for 3 minutes; then place in an oven for 1 hour; The homogenizer was stirred at an ultra-high speed at 20 degrees for 3 minutes, and then put into a 1% polyvinyl alcohol solution and stirred at 600 rpm for 2 hours; centrifuged, collected, and freeze-dried to obtain nanoparticles of the final product.
Embodiment 2
[0055] 1) A mixture of 100 g of sebacic acid in 900 ml of acetic anhydride was refluxed to form acetyl-sebacic acid;
[0056] 2) Mix the 1st) step with the 6-arm polyethylene glycol 19g product terminated with hydroxyl and put it into a flask, and at 180 DEG C. under reduced pressure for 1 hour; ether washed and dried;
[0057] 3) Put 200mg of doxorubicin and 1000mg of polymer from step 2 into 6ml of dimethyl sulfoxide and 14ml of dichloromethane solution for 24 hours; ultrasonicate for 2 minutes; then put in an oven for 2 hours; at minus 10 degrees The medium homogenizer was stirred at an ultra-high speed for 2 minutes, and then put into a 10% cholic acid solution and stirred at 600 rpm for 3 hours; after centrifugation, collection, and freeze-drying, the nanoparticles of the final product were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com