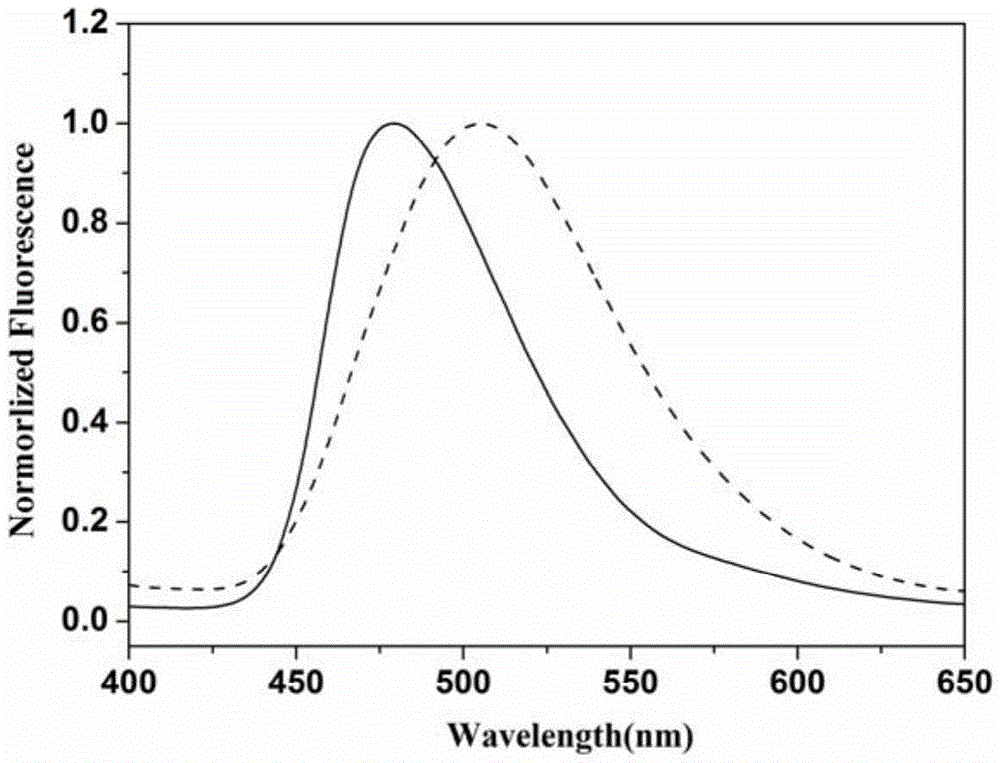
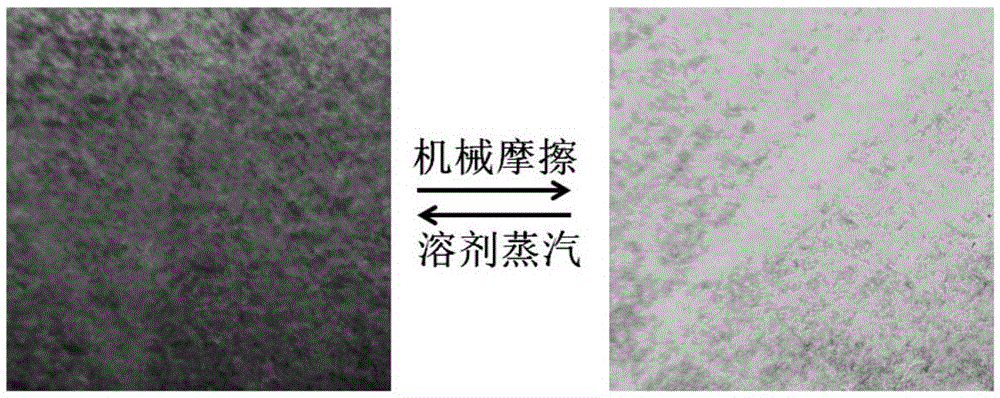
A bispyrene compound with piezochromic properties, preparation method and application thereof
A piezochromic and compound technology, applied in chemical instruments and methods, condensation preparation of carbonyl compounds, organic chemistry, etc., can solve the problem of scarcity of piezochromic materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
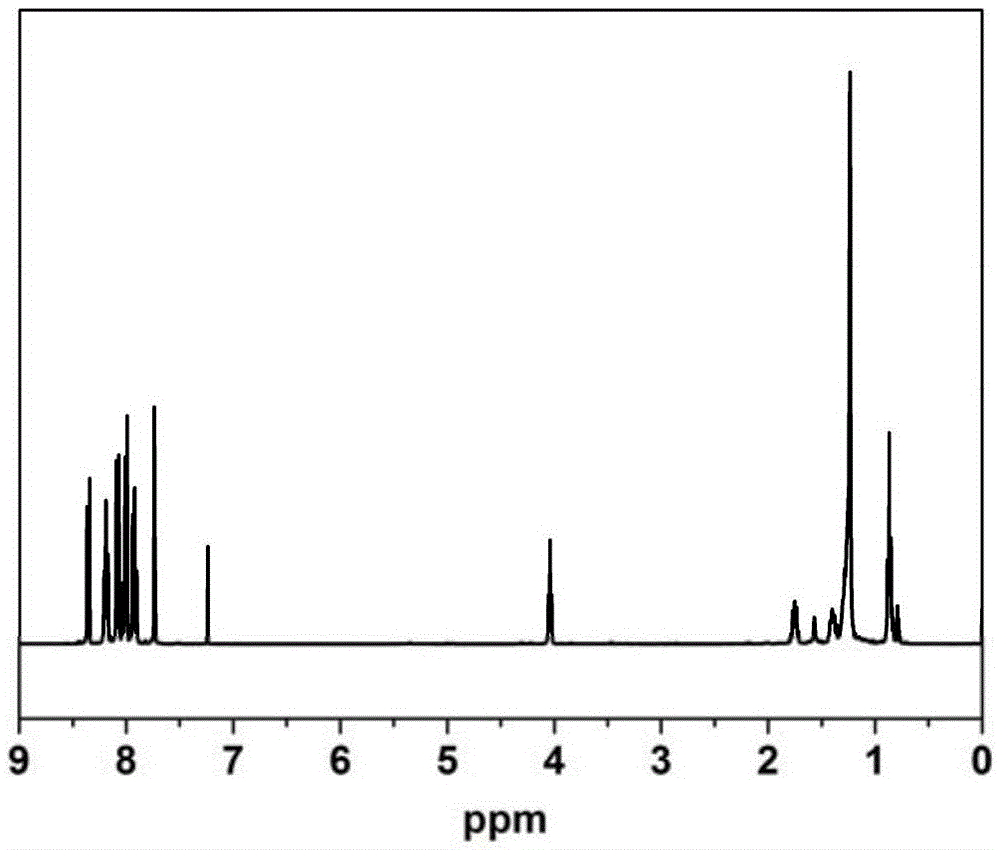
[0077] Take the R structure as C 12 h 25 as an example
[0078] 1. Synthetic method of compound:
[0079] Etherification reaction of compound 1: add 10mmol compound 1 (2.38g), acetone (100mL), 10mmol potassium carbonate (1.37g), compound 10mmolC 12 h 25 Br (2.49g), heated to reflux for 12 hours, concentrated to obtain a crude product, separated by column chromatography, eluted with a mixed solvent of dichloromethane / petroleum ether (volume ratio 2:1), and rotatively evaporated the solvent to obtain a white solid compound 2 (3.07 g, yield: 75.6%).
[0080] Hydrolysis reaction of compound 2: add 30mL ethanol, 60mL water, 5mmol compound 2 (2.03g) and 10mmol potassium hydroxide (0.56g) into the there-necked flask, heat and reflux for 5 hours, add 1M hydrochloric acid dropwise to pH=3 after cooling, Filtration gave white solid compound 3 (1.65 g, yield: 94.3%).
[0081] The acid chlorination reaction of compound 3: in the room equipped with thermometer, dropping funnel, reflu...
Embodiment 2
[0088] Take the R structure as C 3 h 7 as an example
[0089] Etherification reaction of compound 1: add 10mmol compound 1 (2.38g), acetone (100mL), 10mmol potassium carbonate (1.37g), compound 10mmolC 12 h 25 Br (1.23g), heated to reflux for 12 hours, concentrated to obtain a crude product, separated by column chromatography, eluted with a mixed solvent of dichloromethane / petroleum ether (volume ratio 3:1), and rotatively evaporated the solvent to obtain a white solid compound 2 (2.04 g, yield: 73.0%).
[0090] Hydrolysis of Compound 2: Add 30mL of ethanol, 60mL of water, 5mmol of Compound 2 (1.42g) and 10mmol of potassium hydroxide (0.56g) into a three-neck flask, heat to reflux for 5 hours, add dropwise 1M hydrochloric acid to pH=3 after cooling, Filtration gave white solid compound 3 (1.08 g, yield: 96.4%).
[0091] The acid chlorination reaction of compound 3: in the room equipped with thermometer, dropping funnel, reflux condenser (with HCl and SO 2 Add 50mmol of t...
Embodiment 3
[0098] With R structure as (CH 2 CH 2 O) 2 CH 3 as an example
[0099] The etherification reaction of compound 1: add 10mmol compound 1 (2.38g), acetone (100mL), 10mmol potassium carbonate (1.37g), compound 10mmolBr(CH 2 CH 2 O) 2 CH 3 (1.81g), after heating to reflux for 12 hours, concentrated to obtain the crude product, separated by column chromatography, eluted with a mixed solvent of dichloromethane / petroleum ether (volume ratio 3:1), and rotary evaporated the solvent to obtain white solid compound 2 (2.13 g, yield: 71.0%).
[0100] Hydrolysis reaction of compound 2: add 30mL ethanol, 60mL water, 5mmol compound 2 (1.58g) and 10mmol potassium hydroxide (0.56g) into the there-necked flask, heat and reflux for 5 hours, add 1M hydrochloric acid dropwise to pH=3 after cooling, Filtration gave white solid compound 3 (1.21 g, yield: 94.4%).
[0101] The acid chlorination reaction of compound 3: in the room equipped with thermometer, dropping funnel, reflux condenser (wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com