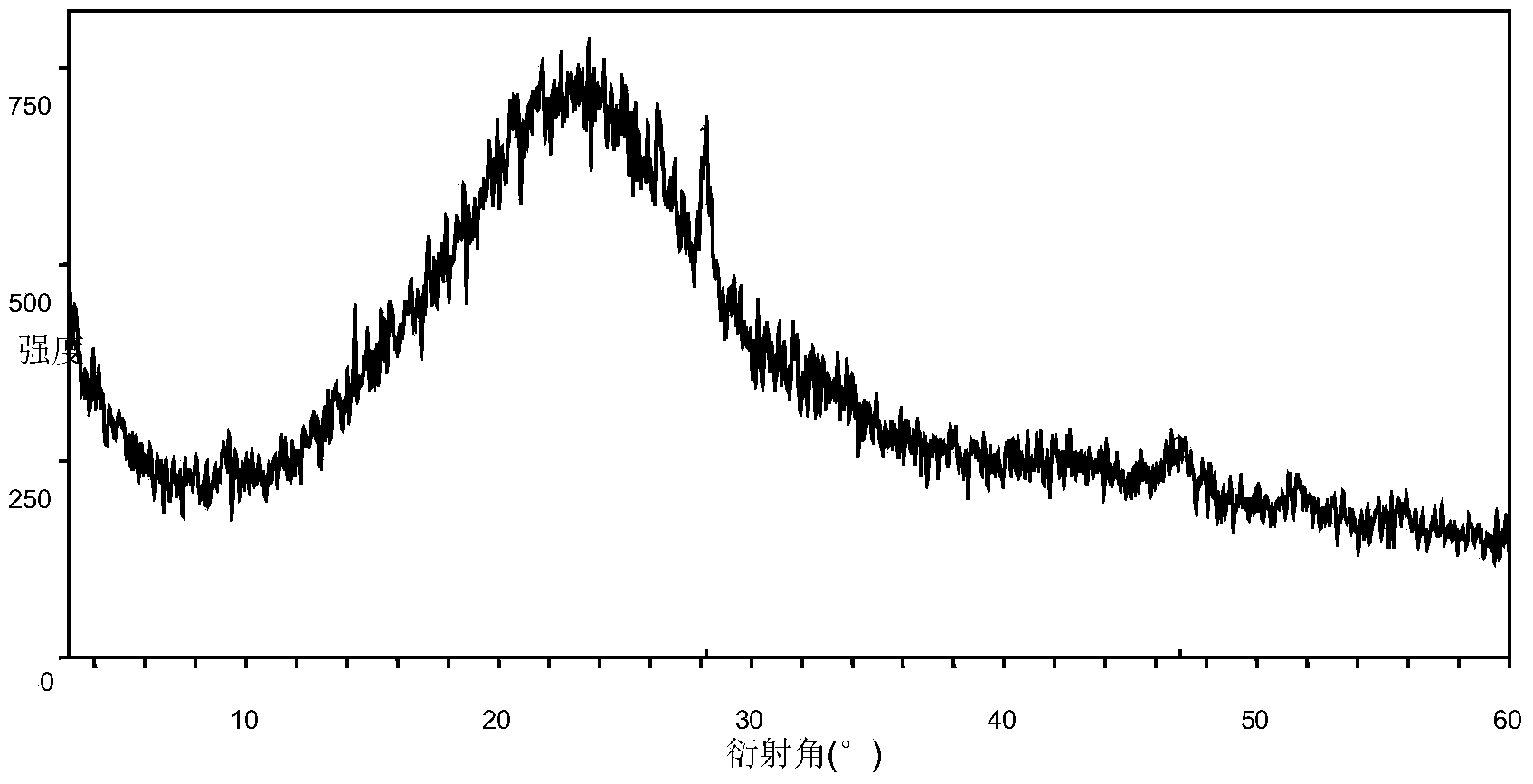
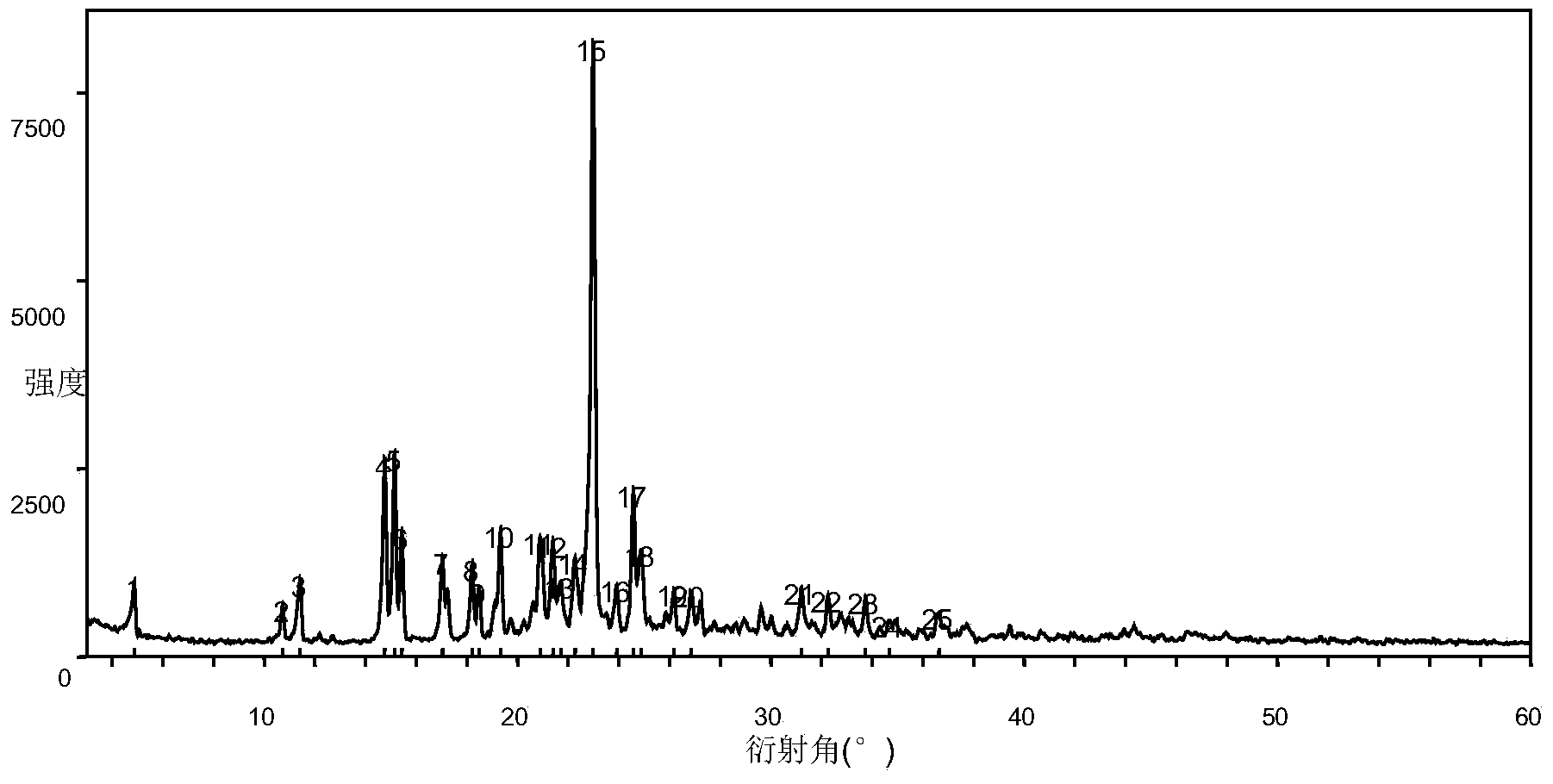
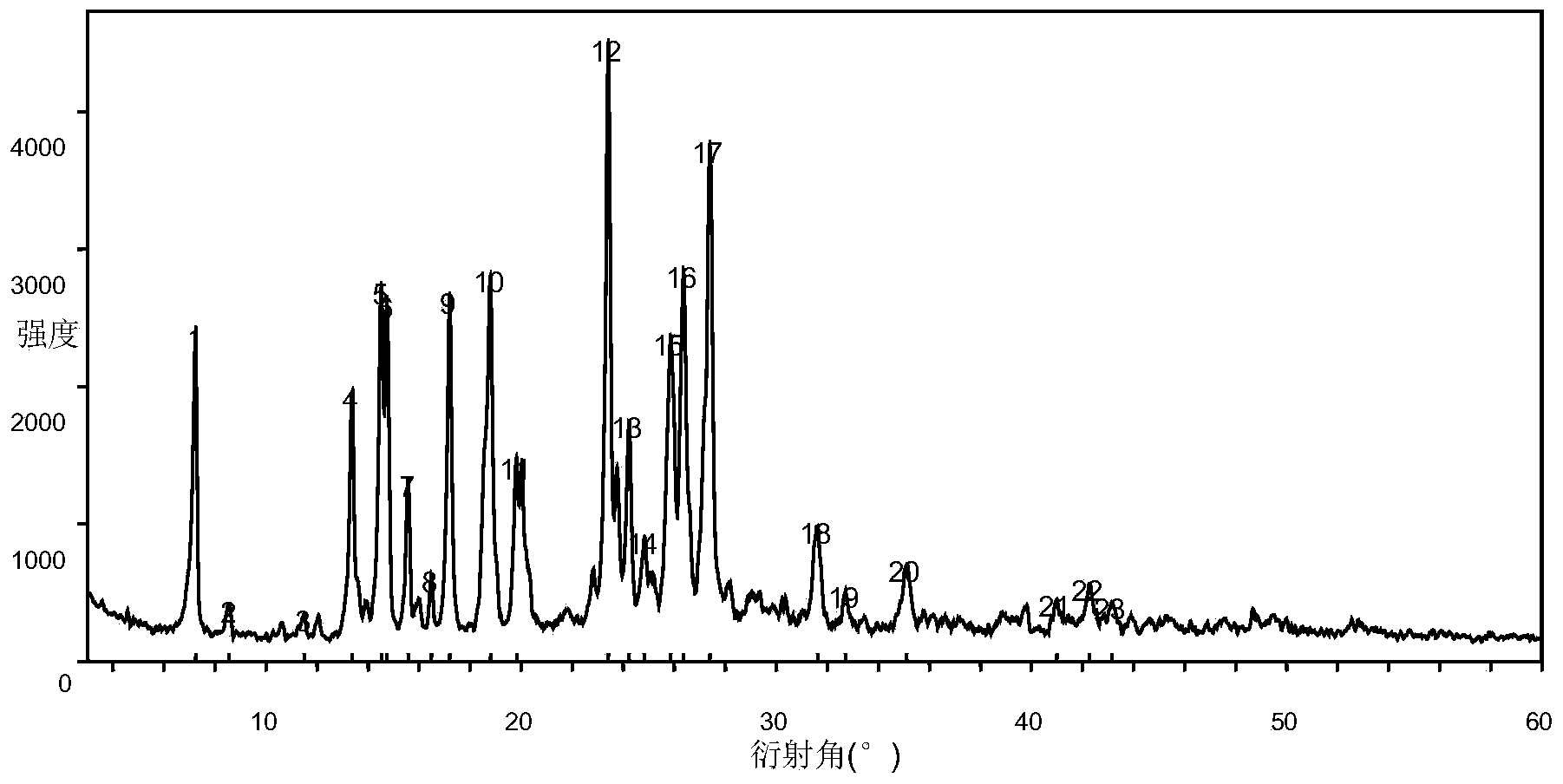
Regorafenib salt, crystal thereof and preparation method of crystal
A technology of regorafenib and regorafenib ethyl sulfonate, applied in the field of regorafenib, can solve the problems of poor solubility of regorafenib monohydrate, limited application and the like, and achieves good stability and product quality. High utilization rate and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Embodiment 1 Preparation of the amorphous crystal form of regorafenib etetinate
Embodiment 11
[0123] Add 1.0 g of regorafenib to 10 ml of ethyl acetate and 1 ml of water, and heat up to 40°C to dissolve. Add 0.33g of 2-hydroxyethylsulfonic acid into the reaction solution, and react at not lower than 40°C for 2 hours. After cooling down to room temperature, no precipitation occurred, and the reaction solution was concentrated to dryness to obtain the amorphous form of regorafenib etetinate.
[0124] 1 H-NMR (400MHz, DMSO): δ2.65-2.68 (t, J = 2.8Hz, 2H), 2.80 (d, J = 4.8Hz, 3H), 3.64 (t, J = 2.8Hz, 2H), 6.71 (broads, 1H), 7.07-7.10 (dd, J 1 =8.8Hz,J 2 =1.2Hz, 1H), 7.26(t, J=2.8Hz, 1H), 7.34-7.37(dd, J 1 =11.6Hz,J 2 =2.8Hz,1H), 7.54(d,J=2.0Hz,1H), 7.63(s,2H), 8.16(m,2H), 8.57(d,J=5.6Hz,1H), 8.80(s,1H ), 8.91(s,1H), 9.57(s,1H).
Embodiment 12
[0126] The same method as in Example 1.1 was adopted, but 10 ml of the used solvent ethyl acetate was replaced by 20 ml of acetone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com