Compound for preparing suvorexant and preparation method thereof
A compound and mixture technology, applied in the field of drug synthesis, can solve the problems of unfavorable industrial mass production, strong irritation, unfriendly environment, etc., and achieve the effect of high ee value, easy purification, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
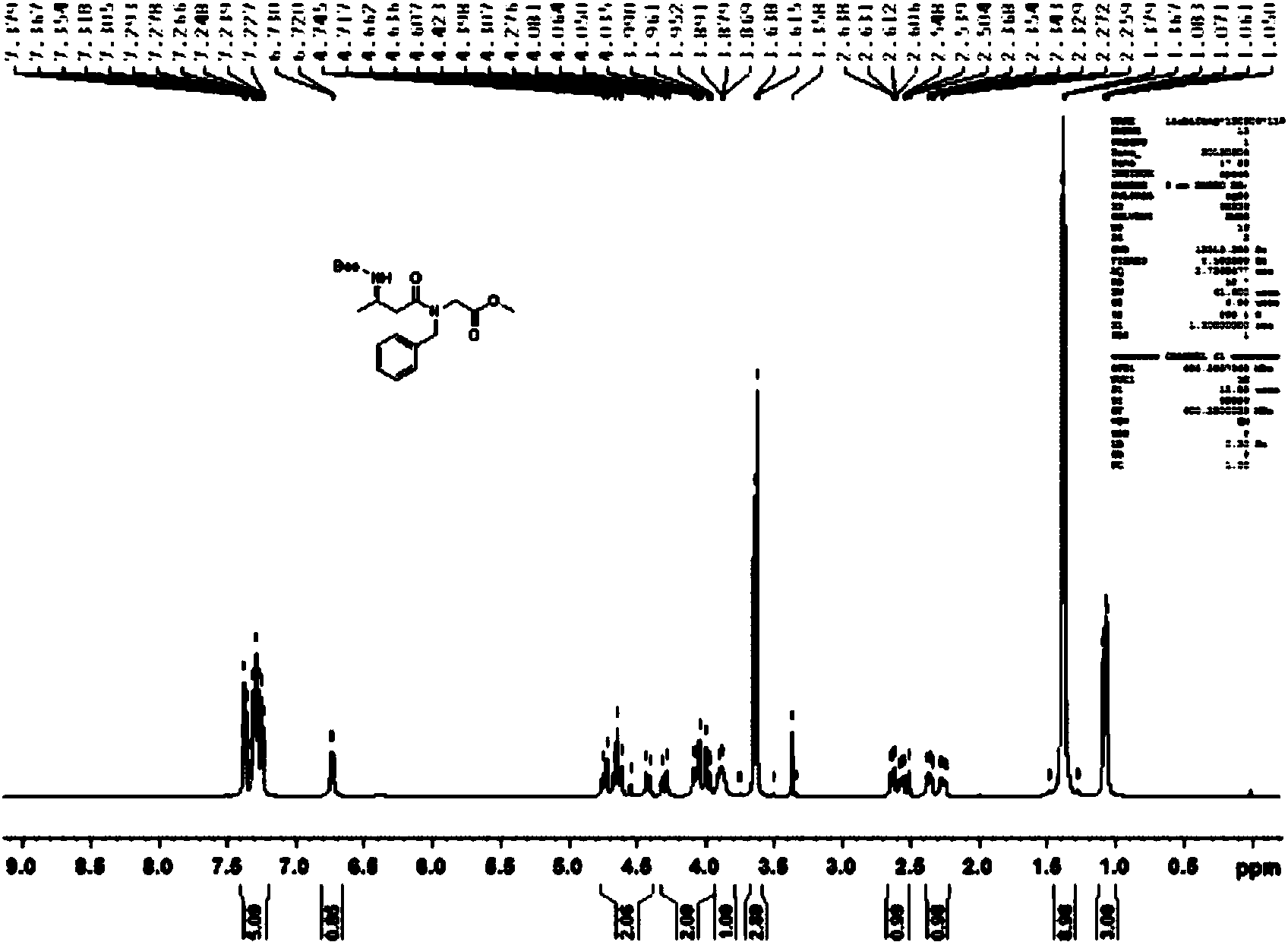
[0064] Synthesis of (R)-methyl-2-(N-benzyl-3-((tert-butoxycarbonyl)amino)butanamido)acetate
[0065]
[0066] Methyl-2-(benzylamino)acetate (20mmol), (R)-3-((tert-butoxycarbonyl)amino)butanoic acid (21mmol), 1-hydroxybenzotriazole (25mmol), dry Add triethylamine (30mmol) into the flask, add 25ml of anhydrous DMF, add EDC (24mmol) under stirring, and react at 10°C for 5h. Add 10% citric acid solution, extract with ethyl acetate, 5% Na 2 CO 3 The organic layer was washed with solution, washed with saturated brine, MgSO 4 Dry, filter and evaporate to dryness, the obtained product is recrystallized with ethyl acetate and petroleum ether (1:2, volume ratio) to obtain (yield 98%, m.p.: 107 ° C, [α] 26 D=21.97 (103.76mg / 20ml, MeOH)). 1HNMR (600MHz, DMSO-d6) δppm7.38-7.23 (m, 5H), 6.73-6.72 (d, 1H), 4.75-4.4 (m, 2H), 4.31-3.95 (m, 2H), 3.89-3.87 ( t,1H),3.64-3.62(d,3H),2.64-2.50(m,1H),2.37-2.23(m,1H),1.38-1.37(d,9H),1.08-1.06(m,3H); ( figure 1 ) MS (ESI) m / z 365.20 ([M+H]+)....
Embodiment 2
[0068] Synthesis of (R)-methyl-2-(N-benzyl-3-((tert-butoxycarbonyl)amino)butanamido)acetate
[0069] Methyl-2-(benzylamino)acetate (20mmol), (R)-3-((tert-butoxycarbonyl)amino)butanoic acid (21mmol), 1-hydroxybenzotriazole (25mmol), carbonic acid Potassium (60mmol) was added to the flask, 50ml of anhydrous dichloromethane was added, CDI (22mmol) was added under stirring, and the reaction was carried out at 20°C for 6h. Add 10% citric acid solution, extract with ethyl acetate, 5% Na 2 CO 3 The organic layer was washed with solution, washed with saturated brine, MgSO 4 Dry, filter and evaporate to dryness, the obtained product is recrystallized with ethyl acetate and petroleum ether (1:2, volume ratio) to obtain (yield 97.5%, m.p.: 107 ° C, [α] 26 D=21.97 (103.76mg / 20ml, MeOH)).
Embodiment 3
[0071] Synthesis of (R)-methyl-2-(N-benzyl-3-((tert-butoxycarbonyl)amino)butanamido)acetate
[0072] Methyl-2-(benzylamino)acetate (20mmol), (R)-3-((tert-butoxycarbonyl)amino)butanoic acid (21mmol), 1-hydroxybenzotriazole (25mmol), hydrogenation Add sodium (24mmol) into the flask, add 50ml of anhydrous acetone, add HOAt (25mmol) under stirring, and react at 30°C for 4h. Add 10% citric acid solution, extract with ethyl acetate, 5% Na 2 CO 3 The organic layer was washed with solution, washed with saturated brine, MgSO 4 Dry, filter and evaporate to dryness, the obtained product is recrystallized with ethyl acetate and petroleum ether (1:2, volume ratio) to obtain (yield 97%, m.p.: 107 ° C, [α] 26 D=21.97 (103.76mg / 20ml, MeOH)).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com