Synthesis method of eto-tilmicosin
A technology based on tilmicosin and tilmicosin, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve problems affecting product quality, low water content in acetone, damage to tilmicosin, etc. problems, to achieve the effect of low water content requirements in solvents, easy filtration, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
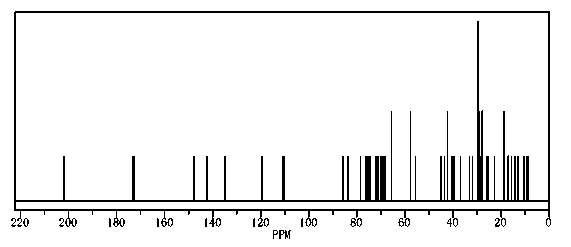
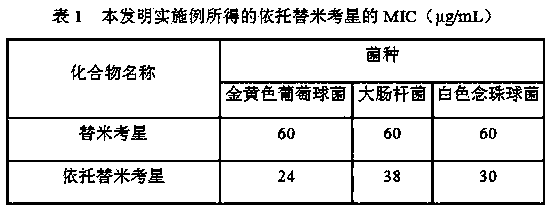
Image
Examples
Embodiment 1
[0032] 1) Add 20g of water and 50g of tilmicosin thiocyanate to 140g of acetone, raise the temperature to 35~38°C, stir for 5 minutes, then add 9g of triethylamine to desulfurize tilmicosin thiocyanate into free tilmicosin Star base, system clarification;
[0033] 2) Add 13.5g propionic anhydride to the system obtained in step 1), react at 35~38°C for 120 minutes to generate tilmicosin propionate, and then filter;
[0034] 3) Add dodecyl sulfate solution to the filtrate obtained in step 2) (dodecyl sulfate solution is prepared as dodecyl sulfate: water = 17g : 90g), and react at 35~38°C 60 minutes to generate eptotilmicosin;
[0035] 4) Add 50g of water to the solution obtained in step 3) at 35~38°C to make the solution turbid, stir for 60 minutes to precipitate a large amount of ettotilmicosin; then add 500g of water to cool down to 5~10°C for 60 minutes to crystallize , centrifuged, and the filter cake was washed with water and dried, and 61 g of ettotilmicosin was isolate...
Embodiment 2
[0037] 1) Add 28g of water and 50g of tilmicosin thiocyanate to 175g of acetone, raise the temperature to 38~42°C, stir for 10 minutes, then add 8g of triethylamine to desulfurize tilmicosin thiocyanate into free tilmicosin Star base, system clarification;
[0038] 2) Add 15g of propionic anhydride to the system obtained in step 1), react at 38~42°C for 120 minutes to generate tilmicosin propionate, and then filter;
[0039] 3) Add dodecyl sulfate solution to the filtrate obtained in step 2) (dodecyl sulfate solution is prepared as dodecyl sulfate: water = 19g : 100g), and react at 38~42°C 40 minutes to generate eptotilmicosin;
[0040] 4) Add 50g of water to the solution obtained in step 3) at 38~42°C to make the solution turbid, stir for 40 minutes to precipitate a large amount of ettotilmicosin; then add 400g of water to cool down to 5~10°C for 60 minutes to crystallize , centrifuged, and the filter cake was washed with water and then dried to isolate 62g ettotilmicosin, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com